thermalequilibriumlabmodifiedportfolio
advertisement

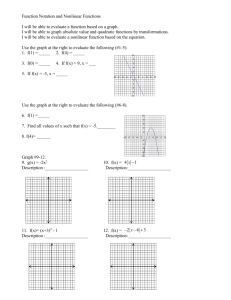
Thermal Equilibrium Lab Activity 1. Go to the following site: http://aventalearning.com/content168staging/2007PhysicsA/labs/thermal/lab.html 2. Follow the instructions at the bottom of the screen (there are four steps…you’ll need to scroll to view them all). Be sure to record the initial and final temperatures of each substance in the “Experiment 1” data table and answer the associated questions. 3. Click on “Experiment 3” at the top of the virtual lab site. Conduct the procedure for this experiment. Record the necessary data, calculations and responses for Experiment 3. 4. Submit this document via the Thermal Equilibrium Lab dropbox. Experiment 1: To study the dependence of heat flow due to the temperature difference between two objects. Use the table below to record your data for Experiment 1. Experiment 1 Cube A Cube B Initial Temperature (°C) Thermal equilibrium temperature (°C) Initial Temperature (°C) Thermal equilibrium temperature (°C) 100 50 0 50 What is the equilibrium temperature of the two masses? Does this seem reasonable? Why or why not? Experiment 3: To study the dependence of heat flow due to the temperature difference between two objects of different composition. Use the table below to record your data for Experiment 3. Experiment 3 Cube A Initial Temperature (°C) Thermal equilibrium temperature (°C) 100 30.74 Composition of cube (roll your cursor over the cube to see what it is made of) copper Cube B Mass of Cube (kg) Initial Temperature (°C) Thermal equilibrium temperature (°C) 1 0 30.82 Composition of cube (roll your cursor over the cube to see what it is made of) Mass of Cube (kg) alluminum Using the data collected for Experiment 3 and the equation Q = mcT, determine the amount of heat given up by Mass A and the amount of heat gained by Mass B. Note: ccopper = 387 J/(kg•°C) caluminum = 899 J/(kg•°C) Record the amount of heat gained/lost by each substance below. How does the amount of heat given up by Mass A compare to the amount of heat gained by Mass B? Does this seem reasonable? Why or why not? Q= energy transfer M= mass of substance C= specific heat capacity Delta T= change in temperature Copper; Q= 1kgX387X(30.74-100)= Aluminum; Q= 1kgX399X(30.82-0)= Compare: 1