CORE 9 EQUILIBRIUM PROBLEMS
EQUILIBRIUM PROBLEMS - ANSWERS
PART A MULTIPLE CHOICE
1. D 2. D 3. C 4. A 5. B 6. D 7. D 8. D 9. B 10. B
NB: Q3 - Reactions involving large energy changes tend not to form equilibria because they are difficult
to reverse.
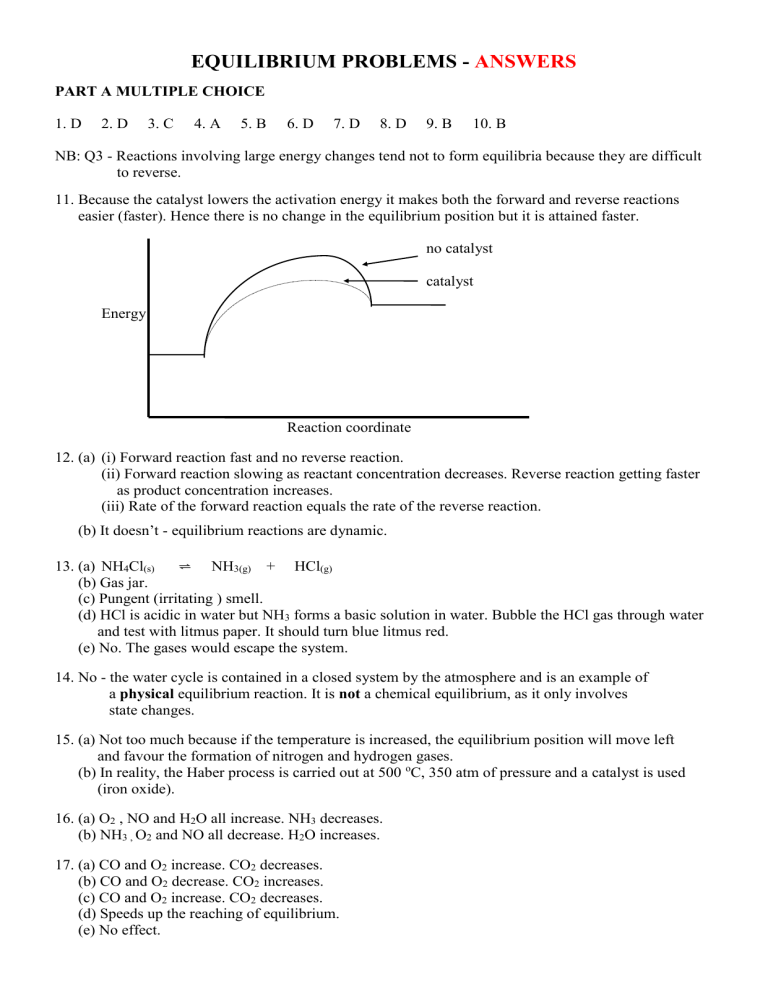
11. Because the catalyst lowers the activation energy it makes both the forward and reverse reactions
easier (faster). Hence there is no change in the equilibrium position but it is attained faster. no catalyst catalyst
Energy
Reaction coordinate
12. (a) (i) Forward reaction fast and no reverse reaction.
(ii) Forward reaction slowing as reactant concentration decreases. Reverse reaction getting faster
as product concentration increases.
(iii) Rate of the forward reaction equals the rate of the reverse reaction.
(b) It doesn’t - equilibrium reactions are dynamic.
13. (a) NH
4
Cl
(s)
⇌
NH
3(g)
+ HCl
(g)
(b) Gas jar.
(c) Pungent (irritating ) smell.
(d) HCl is acidic in water but NH
3
forms a basic solution in water. Bubble the HCl gas through water
and test with litmus paper. It should turn blue litmus red.
(e) No. The gases would escape the system.
14. No - the water cycle is contained in a closed system by the atmosphere and is an example of
a physical equilibrium reaction. It is not a chemical equilibrium, as it only involves
state changes.
15. (a) Not too much because if the temperature is increased, the equilibrium position will move left
and favour the formation of nitrogen and hydrogen gases.
(b) In reality, the Haber process is carried out at 500 o
C, 350 atm of pressure and a catalyst is used
(iron oxide).
16. (a) O
2
, NO and H
2
O all increase. NH
3
decreases.
(b) NH
3 ,
O
2
and NO all decrease. H
2
O increases.
17. (a) CO and O
2
increase. CO
2
decreases.
(b) CO and O
2
decrease. CO
2
increases.
(c) CO and O
2
increase. CO
2
decreases.
(d) Speeds up the reaching of equilibrium.
(e) No effect.
18. (a) Far to the right.
Water molecules will only react to form hydrogen gas and oxygen gas when large amounts
of energy are applied eg. using electrolysis.
(b) Far to the left.
Carbon dioxide gas is very stable and will not form carbon monoxide unless a large amount
of energy is supplied.
19. (a) CH
3
COOH
(aq)
+ H
2
O
(l)
⇌
CH
3
COO
-
(aq)
+ H
3
O
+
(aq)
(b)
(c) If the temperature increases for an endothermic reaction the forward reaction is favoured.
This means that the concentration of acetic acid would decrease and the concentrations of
the acetate ion and hydronium ions would increase.
20. In a 2.0 L flask, 0.7 mol of PCl
3
and 0.5 mol of Cl
2
are allowed to come to equilibrium according
to the equation:
PCl
3(g)
+ Cl
2(g)
⇌
PCl
5(g)
When equilibrium has been reached 0.3 mol of PCl
3
remains.
(a) When equilibrium has been reached how many moles of Cl
2
and PCl
5
are present?
(b) What is the concentration of all three chemical at equilibrium?
(c) Explain why all of the chlorine gas did not react even though the PCl
3
is in excess.
(d) What would happen to this equilibrium if pressure was applied to the system?
20. (a)
Initial
Equilibrium
PCl
0.7
0.3
3
(b) [PCl
3
] = 0.3 /2 = 0.15 mol L
-1
Cl
2
0.5
0.1
[Cl
2
] = 0.1 /2 = 0.05 mol L -1
[PCl
5
] = 0.4 /2 = 0.2 mol L
-1
PCl
0.0
0.4
5
(c) The system is at equilibrium and some PCl
5 will always form some PCl
3
and some Cl
2
.
(d) The equilibrium will shift to the right to form more PCl
5
as there are less particles of gas
on the right side of the equilibrium and this will reduce the effect of the pressure increase.
***************************
DH











