Nuclear Chemistry Equations Worksheet
advertisement
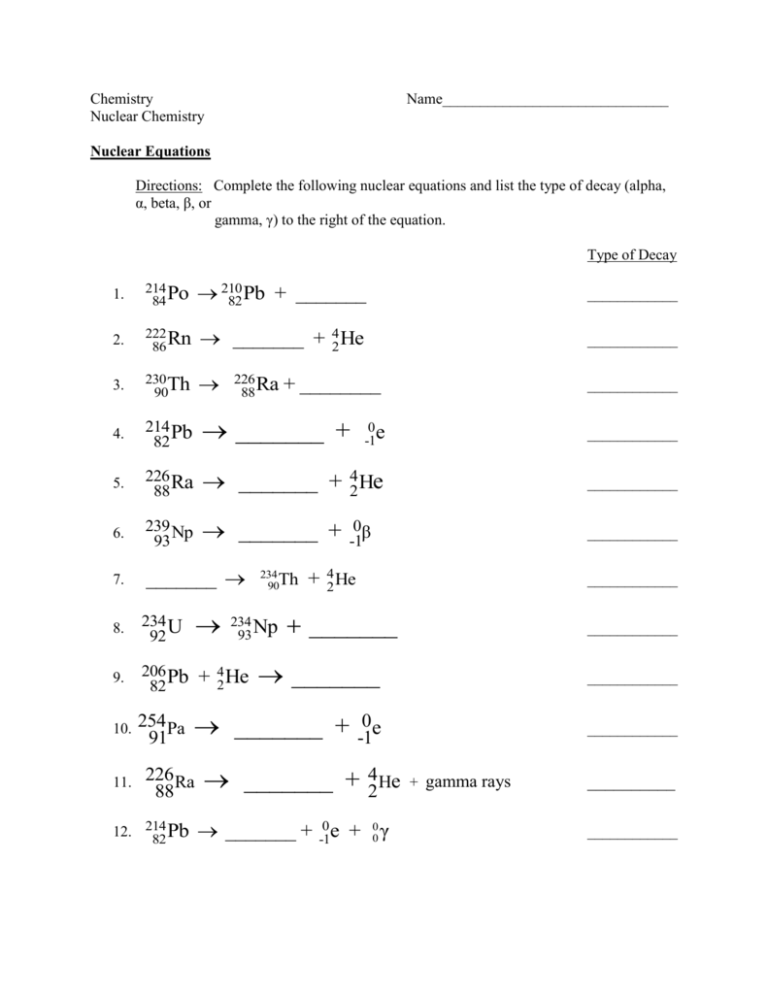
Chemistry Nuclear Chemistry Name______________________________ Nuclear Equations Directions: Complete the following nuclear equations and list the type of decay (alpha, α, beta, β, or gamma, γ) to the right of the equation. Type of Decay 1. 214 Po 84 210 82 Pb + _______ ____________ 2. 222 Rn 86 _______ + 24 He ____________ 3. 230 Th 90 ____________ 4. 214 Pb 82 _______ + 0 -1e ____________ 5. 226 Ra 88 _______ + 42 He ____________ 6. 239 Np 93 _______ + ____________ 7. _______ 8. 234 U 92 9. 206 Pb 82 + 42 He _______ ____________ 10. 254 Pa 91 _______ + -10e ____________ 11. 226 Ra 88 12. 214 82 Pb 226 Ra 88 + ________ 234 90Th 234 Np 93 0β -1 + 42 He ____________ _______ _______ + 4 He 2 _______ + -10 e + 00 γ ____________ + gamma rays __________ ____________ Nuclear Equations II Directions: Complete the nuclear equations by filling in the blank space. 13. 238 92 U 14. 234 90Th ______ + -10 e 15. 234 91Pa ______ + 16. 234 U ______ + 92 17. 234 90Th 230 90Th 226 88 Ra + _______ 0 -1e 4 He 2 + ______ 18. 226 88 Ra 19. 222 86 Rn ______ + 42 α 222 Rn 86 + ______ 20. ______ 21. 214 82 Pb ______ + 22. 214 83 Bi 214 84 Po + ______ 23. 214 84 Po 210 82 Pb + ______ 24. 210 82 Pb 25. 210 83 Bi 214 82 Pb ______ + 26. 210 84 Po 210 84 Po + 24 He 0 -1β 0 -1e + _______ _______ + 42 α 27. This series of nuclear reactions is called a ___________________ _______________ ______________. 28. It shows radioactive Uranium – 238 will form non-radioactive _____________. This process takes approximately _________________________years. 29. Fill in the blank with the isotopic symbol that will complete and balance the following nuclear equations: a. 21 H 21 H 11 H ______ b. 63 29 c. 241 95 Cu 1 1 Am d. ______ e. 64 30 f. 235 92 Zn U H 237 93 2 1 0 1 1 0 e n H 1 0 n 25 13 _______ Al Np _______ 3 2 He 1 0 n _______ 138 56 Ba 301 n _______ 30. Write balanced nuclear equations for the following: a. The alpha decay of neptunium-237 b. The beta decay of bismuth-210 c. The neutron bombardment of tin-120 d. The electron capture of iodine-128 e. The nuclear transmutation of mercury-201 that consists of two alpha decays and 1 beta decay. f. The electron capture of antimony-123 followed by the emission of 2 neutrons. g. Alpha-particle bombardment of plutonium-239 produces a neutron and another isotope. Write the nuclear equation for this reaction and identify the isotope. h. When bombarded with a neutron, lithium-6 produces an alpha particle and an isotope of hydrogen. Write the nuclear equation for this reaction. What isotope of hydrogen is produced? i. With what particle would you bombard sulfur-32 with to produce hydrogen-1 and phosphorus-32? Write the appropriate nuclear equation. j. With what particle would you bombard bismuth-209 to produce astatine-211 and 2 neutrons? Express this reaction in the form of a nuclear equation. 31. Write an equation to describe the alpha decay of a radium-226 nucleus to form a radon nucleus. 32. Write an equation to describe the alpha decay of a radon nucleus to form a polonium-218 nucleus. 33. Write an equation to describe the beta decay of a lead-214 nucleus to form a bismuth-214 nucleus. 34. Write an equation to describe the alpha decay of a uranium-238 nucleus to form a thorium nucleus. 35. Write an equation to describe the beta decay of a thorium-234 nucleus to form a protactimium nucleus. 36. Write the series of equations involving three alpha decays and two beta decays that show the transmutation of a uranium-238 nucleus into a radium-226 nucleus. Isotopic Notation & Nuclear Reactions 37. How many protons and neutrons do each of the following have? a) 241 95 Am b) 63 29 Cu c) 3 __ H d) 40 __ Ar 38. The following are elements with mass numbers. Rewrite the numbers in isotopic notation. a) gold-197 b) magnesium-25 c) lead-210 d) iodine-131 39. Complete the nuclear equation for the alpha emission (decay) for the following isotopes: a) 219 86 Rn c) 234 92 U b) 227 89 Ac d) 253 100 Fm 40. Complete the nuclear equation for the beta emission (decay) for the following isotopes: a) 42 19 K c) 14 6C b) 131 53 I d) 253 98 Cf 41. Complete the following nuclear equations and identify them as either alpha (α) or beta (β) decay. a) 31H 23 He + ______ b) 218 84 Po 214 82 Pb + ______ c) 211 83 Bi 35S d) 16 207 81Tl 35 17 Cl + ______ + ______ 42. Fill in the blanks in the following radioactive decay series: 224 Ra 88 42 He + ______ 42 He + ______ ______ + 212 82 Pb 0 -1e + ______ Changes in the Nucleus 43. Nuclear reactions change the composition of an atom’s _____________________. 44. The attractive force that overcomes the electric repulsion between protons is the ______________ _________________ force. 45. Almost all the atoms you encounter have _________________ nuclei. 46. All nuclei with atomic numbers greater than 83 are __________________________. 47. Alpha, beta and gamma radiation are distinguished by their charge, _________________, and penetrating power. 48. When an atom emits alpha, beta or gamma radiation, it is undergoing _________________ decay. Answer each of the following in the space provided: 49. Why do nuclei need neutrons to be stable? ________________________________________________________________________ ___________________________________________________ 50. In any radioactive decay, the sum of the mass numbers and atomic numbers must be ___________ before and after the reaction. a) greater b) the same c) less d) unpredictable 51. The most dangerous form of radiation to the human body is a) beta radiationb) gamma radiation c) alpha radiation d) They are equally dangerous 52. To be stable, atoms with more than 20 protons need increasingly more a) neutrons than protons b) electrons than protons c) electrons than neutrons d) protons than neutrons 53. The sun produces energy by means of a) gamma radiation b) beta decay 222 56. 226 88 Ra 86 Rn + ______ 57. 14 6C 27 Al 58. __ 14 7N + 4 __ He c) alpha decay d) nuclear fusion + ______ 12 C 6 + ______ Write a nuclear equation for the following reactions: 59. alpha decay of polonium-210 _______________________________________________________ 60. beta decay of tritium ( 31H ) _________________________________________________________ 61. alpha decay of thorium-230 ________________________________________________________ 62. alpha decay of 231 91 Pa _____________________________________________________________ 63. What particle bombards Beryllium-9 if it forms carbon-12 and neutrons? _________________________________________________________________________ 64. beta decay of 165 61 Pm _____________________________________________________________ 65. What isotope is bombarded with alpha particles to produce oxygen-17 and hydrogen-1? ________________________________________________________________________ 66. alpha decay of 146 62 Sm ____________________________________________________________ 67. beta decay of 198 85 At _____________________________________________________________ 68. bombardment of boron-10 with neutrons to produce Li-7 and another particle _______________________________________________________________ 69. beta decay of 152 54 Xe ________________________________________________________________ Nuclear Bombardment Problems 70. Bombardment of aluminum-27 by alpha particles produces phosphorus-30 and one other particle. Write the nuclear equation for this reaction and identify the other particle. 71. When bombarded with neutrons, cobalt-59 is converted to cobalt-60. What is the nuclear equation for this reaction? 72. Neutron bombardment of plutonium-239 yields americium-240 and another particle. Write the nuclear equation and identify the other particle produced. 73. Alpha-particle bombardment of plutonium-239 produces a neutron and another isotope. Write the nuclear equation for this reaction and identify the isotope. 74. When bombarded with neutrons, lithium-6 produces an alpha particle and an isotope of hydrogen. Write the nuclear equation for this reaction. What isotope of hydrogen is produced? 75. With what particle would you bombard sulfur-32 to produce hydrogen-1 and phosphorus32? Write the appropriate nuclear equation. 76. With what particle would you bombard bismuth-209 to produce astatine-211 and 2 neutrons? Express this reaction in the form of a nuclear equation. 2 77. 1 H ________ 24 He 147 N _______ 11H 4 78. 2 ½ Life Notes Definition: The time it takes for _________________ of the atoms in a ______________________ material to _______________. This time can range from _________sec to __________________ of years. Examples: 79. Carbon-14, remain? ½ life = 5730 years. If you start with 128g, how long until 8g TIME AMOUNT 80. If the ½ life of element x is 100. years, how much of the isotope will remain after 500 years if you start with 128g? TIME AMOUNT 81. 20.g of a radioisotope with a ½ life of 200 hours is prepared in a nuclear reactor. How much will remain after 600. hours? TIME AMOUNT Half-life Problems 82. The half-life of cesium-137 is 30.2 years. If the initial mass of a sample of cesium-137 is 1.00 kg, how much will remain after 151 years. 83. With a half-life of 28.8 years, how long will it take for 1.00g of strontium-90 to decay to 0.125g? 84. A 1.000-kg block of phosphorus-32, which has a half-life of 14.3 days, is stored for 100.1 days. At the end of this period, how much phosphorus-32 remaining 85. There are 3.29 g of iodine remaining in a sample originally containing 26.3 g of iodine126. The half-life of iodine-126 is 13 days. How old is the sample? 86. A sample of air from a basement is collected to test for the presence of radon-222, which has a half-life of 3.8 days. However, delays prevent the sample from being tested until 7.6 days have passed. Measurements indicate the presence of 6.5 g of radon-222. How much radon-222 was present in the sample when it was initially collected? 87. The half-life of carbon-14 is 5730 years. If a sample originally contained 3.36 g of C-14, how much is present after 22,920 years? 88. Gold-191 has a half-life of 12.4 hours. After one day and 13.2 hours, 10.6 g of gold-191 remains in a sample. How much gold-191 was originally present in the sample?









