PGLNext Letter of Medical Necessity
advertisement

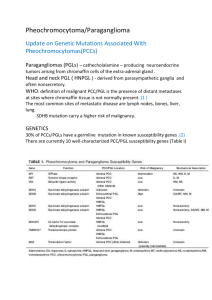
LETTER OF MEDICAL NECESSITY FOR HEREDITARY PHEOCHROMOCYTOMA/PARAGANGLIOMA GENETIC TESTING (PGLNext) Date: Date of service/claim To: Utilization Review Department Insurance Company Name, Address, City, State Re: Patient Name, DOB, ID # ICD-9 Codes: (quick reference as suggestions: 194.6 malignant neoplasm of aortic body and other paraganglia; 227.6 benign neoplasm of aortic body and other paraganglia; 237.3 neoplasm of uncertain behavior of paraganglia; V18.19 family history of other endocrine and metabolic diseases) This letter is in regards to my patient and your subscriber, First, Last Name to request full coverage of medically-indicated genetic testing for hereditary paraganglioma/pheochromocytoma to be performed by Ambry Genetics Corporation. Paragangliomas (PGL) and pheochromocytomas (PCC) are endocrine tumors thought to have a hereditary component in up to 40% of cases. Those with hereditary PGL/PCC are at risk for multiple PGL/PCC, some of which have a high risk of becoming malignant. Those with hereditary conditions related to PGL/PCC have an increased lifetime risk of developing tumors and/or cancers (such as up to a 70% risk of developing renal cancer in those with von Hippel-Lindau disease, and up to a 100% risk of developing medullary thyroid cancer in those with multiple endocrine neoplasia type 2). Some of these gene mutations also increase the lifetime risk for additional cancers/tumors (like pancreatic tumors, hemangioblastomas, neurofibromas, optic gliomas, acoustic neuromas, and other neuroendocrine tumors).1,2,3 Significant aspects of my patient’s personal and/or family medical history that suggest a reasonable probability of hereditary PGL/PCC are below: Based on the above history, I am requesting coverage for this test (PGLNext), which analyzes 12 high-risk genes associated with increased risks for PGL/PCC: FH, MAX, MEN1, NF1, RET, SDHA, SDHAF2, SDHB, SDHC, SDHD, TMEM127, VHL. Due to the history stated above, there is a reasonable probability of detecting a mutation in my patient. The clinical overlap with mutations in the abovementioned genes makes this multi-gene test the most efficient and cost-effective way to analyze these genes.4 Therefore, germline genetic testing is warranted.1,2,4 This genetic testing will help estimate my patient’s risk to develop tumors/cancer. It will also directly impact my patient’s medical management. An aggressive approach following guidelines is indicated in those that carry a mutation found by this test. Management guidelines to reduce the morbidity and mortality associated with these tumors/cancers may include: Consideration of CT/MRI-based screening/technologies Annual biochemical screening More prompt removal of tumor due to increased malignant potential (especially if a SDHB mutation is found by this test) Prophylactic thyroidectomy Annual ophthalmology and audiology examinations Other: ____________________________________ Due to the tumor/cancer risks associated with these mutations and the interventions available, this genetic testing is medically indicated. As such, I am ordering this testing as medically necessary and affirm that my patient has provided informed consent for genetic testing. A positive test result would confirm a genetic diagnosis and/or risk in my patient, and would ensure my patient is being managed appropriately. I am specifying Ambry Genetics Corporation because this laboratory has highly-sensitive and cost-effective testing for hereditary PGL/PCC, along with a large database of previously tested patients to ensure highly validated, accurate, and informative test interpretation. I recommend that you support this request for coverage of diagnostic genetic testing for hereditary PGL/PCC in my patient. Genetic testing can take several weeks to complete, and the laboratory will not bill until testing is concluded. Therefore, I am requesting that the authorization be valid for 3 months. Thank you for your time, and please don’t hesitate to contact me with any questions. Sincerely, Ordering Clinician Name (Signature Provided on Test Requisition Form) (MD/DO, Clinical Nurse Specialist, Nurse-Midwives, Nurse Practitioner, Physician Assistant, Genetic Counselor*) *Authorized clinician requirements vary by state Test Details CPT codes: 81228x1, 81403x1, 81404x1, 81405x1, 81406x1, 81408x1 Laboratory: Ambry Genetics Corporation (TIN 33-0892453 / NPI 1861568784), a CAPaccredited and CLIA-certified laboratory located at 15 Argonaut, Aliso Viejo, CA 92656 References: 1. Fishbein L, et al. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol. 2013 May;20(5):1444-50. 2. Lenders JW, et al. Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014 Jun;99:1915–42. 3. Giusti F, Marini F, Brandi ML. Multiple Endocrine Neoplasia Type 1. 2005 Aug 31 [updated 2015 Feb 12]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015. 4. Karasek D, et al. An update on the genetics of pheochromocytoma. J Hum Hypertens. 2013 Mar;27(3):141-7.