7.2 change summary
advertisement
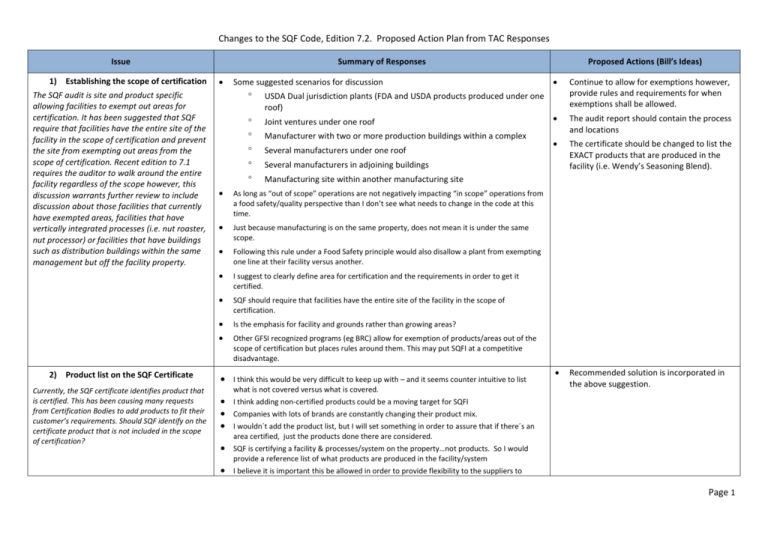
Changes to the SQF Code, Edition 7.2. Proposed Action Plan from TAC Responses Issue 1) Establishing the scope of certification The SQF audit is site and product specific allowing facilities to exempt out areas for certification. It has been suggested that SQF require that facilities have the entire site of the facility in the scope of certification and prevent the site from exempting out areas from the scope of certification. Recent edition to 7.1 requires the auditor to walk around the entire facility regardless of the scope however, this discussion warrants further review to include discussion about those facilities that currently have exempted areas, facilities that have vertically integrated processes (i.e. nut roaster, nut processor) or facilities that have buildings such as distribution buildings within the same management but off the facility property. 2) Product list on the SQF Certificate Currently, the SQF certificate identifies product that is certified. This has been causing many requests from Certification Bodies to add products to fit their customer’s requirements. Should SQF identify on the certificate product that is not included in the scope of certification? Summary of Responses Some suggested scenarios for discussion USDA Dual jurisdiction plants (FDA and USDA products produced under one roof) Joint ventures under one roof Manufacturer with two or more production buildings within a complex Several manufacturers under one roof Several manufacturers in adjoining buildings Manufacturing site within another manufacturing site As long as “out of scope” operations are not negatively impacting “in scope” operations from a food safety/quality perspective than I don’t see what needs to change in the code at this time. Just because manufacturing is on the same property, does not mean it is under the same scope. Following this rule under a Food Safety principle would also disallow a plant from exempting one line at their facility versus another. I suggest to clearly define area for certification and the requirements in order to get it certified. SQF should require that facilities have the entire site of the facility in the scope of certification. Is the emphasis for facility and grounds rather than growing areas? Other GFSI recognized programs (eg BRC) allow for exemption of products/areas out of the scope of certification but places rules around them. This may put SQFI at a competitive disadvantage. I think this would be very difficult to keep up with – and it seems counter intuitive to list what is not covered versus what is covered. Proposed Actions (Bill’s Ideas) Continue to allow for exemptions however, provide rules and requirements for when exemptions shall be allowed. The audit report should contain the process and locations The certificate should be changed to list the EXACT products that are produced in the facility (i.e. Wendy’s Seasoning Blend). Recommended solution is incorporated in the above suggestion. I think adding non-certified products could be a moving target for SQFI Companies with lots of brands are constantly changing their product mix. I wouldn´t add the product list, but I will set something in order to assure that if there´s an area certified, just the products done there are considered. SQF is certifying a facility & processes/system on the property…not products. So I would provide a reference list of what products are produced in the facility/system I believe it is important this be allowed in order to provide flexibility to the suppliers to Page 1 Changes to the SQF Code, Edition 7.2. Proposed Action Plan from TAC Responses exclude products and allow for clear communication to customers on the certificate so that they fully understand that though the excluded product is manufactured at the certified site, the product is not covered. 3) Seasonal Product Currently, there is no variability for season product for the recertification or surveillance audit. The SQF will need to include criteria as to how the audit should be managed for seasonal products. Provisions should also state that the audit needs to take place during operational periods and a limit on how far out in time you can go. Is the process or a specific product seasonal? I mean does it change in a way that all SQF system changes? If not this doesn´t make sense. Yes, SQF should include criteria on how the audit should be managed for seasonal production. Seasonality should be taken into account for surveillance in order to ensure production is observed. 4) Switching CBs The current requirement is that sites that are under a surveillance audit are not allowed to switch to a different CB until the surveillance audit is completed. The intent is to have consistency in the perspective of the facility’s non-conformances and corrective actions. The issue is that that sites that want to opt out of their audit look to SQF to exempt them from this requirement and when not granted would choose to lose certification rather than continue with the existing CB. Should SQF revert to the requirements under edition 6, and remove all requirements as to when the site can switch CBs? Recommend adding a new section for season suppliers to include: 1) Resetting the recertification audit date 2) When to audit if more than one season product 3) When to conduct a surveillance audit. I understand this is an issue when facilities are ‘shopping for a better score’ or when Change the surveillance audit form not a surveillance audit is trying to be avoided but I don’t know that you can prohibit a issuing a score to having a new score and supplier from switching – isn’t it a bit like trying to prohibit free trade? rating based off the results of the surveillance audit. The surveillance audit will Would it be possible to allow them switch CBs while under a surveillance audit but not change the recertification date. require the new CB to verify that all non-conformances and corrective actions were addressed from the previous CB? Allow for suppliers to switch under specific conditions: Perhaps we can allow them to switch CB’s but the surveillance requirement remains 1 – For due cause intact. That way, if they have an issue with the CB or auditor, they can switch and the surveillance audit requirement remains intact. 2 - After there is documented evidence If they want to change, at least SQF should define the new CB assigned to the site, provided by the site that they have and the surveillance audit and status should continue with the new CB. followed the CBs internal process to Stay the course and do not allow sites that are under a surveillance audit to switch address the due cause CB”s. This not only drives consistency of non-conformances and corrective actions. 3 - Site provide a copy of their last 1-2 It also discourages “CB shopping”. (min.) SQF audit reports to the new CB Switching CBs should be allowed, but only: 1 – For due cause 2 - After there is documented evidence provided by the site that they have followed the CBs internal process to address the due cause 3 - Site provide a copy of their last 1-2 (min.) SQF audit reports to the new CB 5) Understanding the SQF Practitioner per site If we are going ahead with the ‘Advanced Practitioner’, we need to reference in the Code. Also, what do we do with the small sites and sub-sites? It is important to sort out this I believe that each site regardless of size needs to have a trained SQF practitioner on site. I agree. In the absence of a practitioner at a small site or sub-site, than a responsible employee shall be designated by the corporate practitioner with sufficient oversight of the operations I like the new provision and allowing for continuous improvement and learning Retain this requirements Guidance for the supplier and the auditor is provided in the Guidance document for Module 2 SQF Advanced Practitioner will not be added to 7.2 of the code. Page 2 Changes to the SQF Code, Edition 7.2. Proposed Action Plan from TAC Responses issue for clarification for the sites. under the standard If this is not going to be a requirement (advanced practitioner) then shouldn´t been referenced. Yes, pls include the advanced practitioner in the code. Are the small sites and sub-sites part of a multisite? Advanced Practitioner credential or training course should be documented as a definition in the SQF Code only. If it’s written into 2.1.2.5 it is very likely that it will be misinterpreted by auditors, practitioner or consultants who will start insisting that the SQF Practitioner at the site must have this credential. I strongly support the requirement of SQF Practitioner per site (including sub-sites). The SQF Code makes it easy for any qualified individual within the facility to become the SQF practitioner. 6) Viewing results of non-conforming product Based off the findings from Sunland, it was discovered that product was shipped out that did not meet microbiological specification. This prompted us to include auditor guidance under section 2.4.6-Non-conforming product- that provides direction for the auditor to request reviewing documentation as to how product that did not meet specification was handled. Moving forward how should SQF address this in the SQF Code that requires the finished product or raw materials that do not meet specifications be recorded and actioned. 7) Adjusting the code requirement for compressed air In recent discussions with Parker Balston, filtration products, they have suggested alternative language to clarify the intent of the compressed air quality element. There has been a lot of confusion with this element and this may need to get revisited. I agree absolutely – out of spec materials need to have follow up investigation documented, product disposition recorded and corrective actions and preventive measures, if needed. One way would be to have the supplier perform a mock-recall on the product during the audit. At level 2, they should review documentation for non-conforming to the FS plan. This should already be part of the HACCP review FDA is going to be looking at environmental testing and product pathogen results. Yes, SQF should require that finished product and/or raw materials that do not meet specifications be recorded and actioned. This requirement is addressed in 2.4.6.2: Non-conforming Product or Equipment: Quarantine records, and records of handling, corrective action, or disposal of non conforming product or equipment shall be maintained. Checking and asking for records for nonconforming records is also described in guidance for this section. SQF will continue to emphasize in auditor training and professional update training. Nonconforming product is currently addressed in 2.4.6, managed so as to minimize potential for inadvertent/improper use as was the case with Sunland Co I agree this is very confusing. Compressed air contamination can occur in numerous ways and if undetected, it can contaminate final product. Waiting for proposed changes from Parker Balston. Most compressed air systems have oil traps and filters to protect the product. It would help to have additional guidance as to what the expectation is for suppliers It is wise for SQF to revisit the compressed air quality language and make it clear I agree on the language, very confusing I would like to see proposed language for this. I feel that this is best addressed in the guidance document. 8) Viewing regulatory reports and warning letters I believe this belongs in the “Business” section of the code – Part A but not in the code itself It would make it easier if incorporated into the actual code. I would suggest placing it in 2.4.1 Add requirements to: 2.4.1.3- SQFI and the CB shall be Page 3 Changes to the SQF Code, Edition 7.2. Proposed Action Plan from TAC Responses With the many regulatory inspections and warning letters SQF has incorporated in the requirements under Part A, section 5.3 that requires the supplier to inform the CB and SQF of any food safety event that requires public notification (such as Class I, Class II and Regulatory Warning letters). However, does this require SQF to have this added into the code and if so, where would this be required. Food Legislation (Regulation) (M) Only in the countries with this regulatory requirements. Yes, notification of recalls and warning letters should be in the code….not sure where. This item is already addressed in 2 places in the SQF Code (Part A, 5.3 and Part B, 2.1.5.1). I do not see a need to address it further in the Code. Perhaps it should be addressed in the guidance document under Part B, 2.1.5.1. 9) Multi-site requirements The multi-site requirement may be changing with the publication of the Storage and Distribution guidance document and SQF will need to reconsider how this would apply to the code. 10) Use of the Quality Shield There are large corporations that would like to have one Quality Shield used for all facilities. SQF should consider changing the Quality Shield requirement to allow for multiple facility use of the same logo and number. 11) Adding modules Develop a module for brokers. Develop a module for warehousing and retailers This will be very big and tough to get our arms around. I am anxious to see where GFSI goes with this. Careful consideration should be paid to multisite requirements to ensure the entire food system is contained in the certification. What is in the Storage and Distribution guidance that would be of concern? Lets see what GFSI comes up with I was told a few years ago by a GFSI Board member that GFSI discourages the use of any labeling that indicates a product may be ‘safer’ than another. This makes sense but I’d have concerns about the shield being inappropriately applied to a facility that is not SQF certified. This could be good, but does this mean that if one facility does poorly on an audit and status is removed, that the Quality Shield usage would need to be removed from all? Yes, one Quality Shield should be available for all facilities within a large company. No comment. This makes sense I believe flexibility could be applied for this and a corporate master number be assigned. I propose the corporate number be assigned through the database and tied to the certification number for the sites so that in a public query of the database the sites are identified FONA International would be happy to participate on development of these models if you need working group members. The broker and warehouse modules are going to be very important for these entities to comply with FSMA. Especially for brokers under the Preventative Controls for Supplier Verification. Yes, pls add modules for brokers, warehousing & retailers. I support this notified within 24 hours in instances in which a supplier receives a regulatory warning letter. 2.6.1.3.iv: SQFI and the CB shall be listed as an essential body and be notified in instances in which there is a food safety incident of public nature. Work with CBs to provide examples and further clarification for regulatory warning letters GFSI will be revising this requirement. SQF should wait for this guidance and make the necessary modifications. Recommend making changes to Appendix 3., schedule 1: Corporate Shields can be issued for organizations wishing to display the SQF Quality Shield at the corporate site. SQF will issue one shield number to be used at the corporate site. The other, SQF certified sites, will be required to use their assigned Quality Shield with their unique number. Will be adding the Broker module based off of TAC feedback. Retail module will wait for GFSI guidance. Page 4 Changes to the SQF Code, Edition 7.2. Proposed Action Plan from TAC Responses 12) Unannounced audits Expressed during the closing session for GFSI, the use of unannounced audits will be coming to GFSI in the future. This is something that should be discussed as how unannounced audits should be used within SQF. 13) Other areas for discussion I believe this will be challenging for CBs to manage and especially difficult for small manufacturers. Fully support This is needed and something that I support. I like the idea discussed as one audit in three with reasonable advance warning to make sure the facility is operating, not undergoing another audit with a customer or other exemptions that need to be spelled out. Need to define goals and consequences YES! YES! YES! Unannounced audits should be used within SQF. This is essential to renew confidence in GFSI and SQF Today the industry has gotten too good at “prettying itself up”. Unannounced audits would eliminate this phenomenon. All SQF certified sites should be audit ready 7x24. Growers – this is a challenge due to harvest timing especially if grower has one variety of production. Unannounced audits are currently an option under BRC, Two respondents (Holly, Joan) mentioned ‘management commitment’: Management Commitment and Food Safety Culture seem to be one in the same in my mind and I know the Guidance Document Working Group sent a message back to the GFSI Board that a working group should be formed to determine how to measure Food Safety Culture. I believe that the efforts toward a global food handler curriculum and good training practices will be one of the ways to measure management commitment and the culture within a facility Management Commitment – SQF must continue its focus and increased requirements re: management commitment. Captured in separate document Management commitment is discussed in detail in the SQF Guidance for module 2. Page 5











