lab three
advertisement

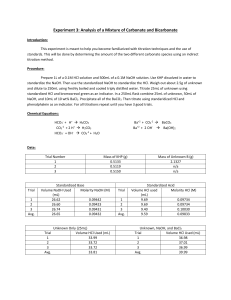
Nicole Lucas Experiment Three: Analysis of a Mixture of Carbonate and Bicarbonate Introduction: The purpose of this lab is to familiarize ourselves with the technique of titration. In this lab, we will be conducting direct and indirect acid base titrations using primary and secondary standards. Our goal will be to determine the amount of two different carbonate species. Procedure: Obtain one unknown containing a mixture of sodium bicarbonate and sodium carbonate. Store it in the dessicator until needed. Prepare 1 liter of 0.1M HCl and 500 mL of 0.1M NaOH. Using primary standard grade KHP, weigh enough KHP to require at least 25 mL of titrant. Standardize the 0.1M NaOH. Repeat this procedure until three good trials are obtained. Use the standardized base to standardize the 0.1M HCl. Repeat this until three good trials are obtained. Accurately weight 2.0-2.5 g of unknown into a 250 mL volumetric flask. Dilute to the mark with freshly boiled and cooled triply distilled water. Pipet a 25.00 mL aliquot of the unknown into a 250 mL Erlenmeyer flask and titrate with standardized HCl using bromocresol green to determine the endpoint. Repeat until three good trials are obtained. Pipet a 25.00 mL aliquot of unknown and a 50.00 mL aliquot of standardized NaOH into a 250 mL Erlenmeyer flask. Add 10 mL of 10 wt% BaCl2, swirl to precipitate all BaCO3, then immediately titrate with standardized HCl using phenolphthalein indicator. Repeat until three good trials are obtained. Chemical Equations: + HCO− 3 + 2H → H2 CO3 + CO2− 3 + 2H → H2 CO3 2− − HCO− 3 + OH → CO3 + H2 O Ba2+ + CO2− 3 → BaCO3 (s) 3+ Ba + 2OH − → Ba(OH)2(s) Data: Standardizing NaOH: Mass KHP (g) Sample 1 Sample 2 Sample 3 Average Volume NaOH (mL) 0.5103 Molarity NaOH (mol per liter) 26.75 0.09341 0.5107 26.52 0.09430 0.5102 25.90 0.09646 0.5104 26.39 0.09472 Standardizing HCl Volume NaOH (mL) Sample 1 Sample 2 Sample 3 Average Volume HCl (mL) Molarity HCl (mol per liter) 25 28.3 0.0825 25 27.1 0.0870 25 25.1 0.0961 25 26.83 0.0885 Determining Unknown with Standardized Acid Sample 1 Sample 2 Sample 3 Average Volume Unknown Volume HCl (mL) moles HCO3(mL) 25.0 35.02 0.002889 25.0 41.80 0.003636 25.0 37.60 0.003613 25.0 38.14 0.003379 Determining Unknown with Standardized Acid and BaCl2 Sample 1 Sample 2 Sample 3 Average Volume Unknown Volume HCl (mL) moles CO3^2(mL) 25.0 33.20 25.0 33.80 0.001852 25.0 33.61 0.001433 25.0 33.54 0.001512 Wt% Sample 1 Sample 2 Sample 3 Average Standard Deviation Weight Percent HCO37.82 9.84 9.77 9.14 1.15 Calculations: Finding amount of KHP to use: Molarity of NaOH: 0.001252 Weight Percent CO3^23.33 4.92 3.81 4.02 0.815 Molarity of HCl: Moles HCO3-: M HCl * Volume HCl = mol HCO30.0825M HCl * 0.03502 L HCl = 0.002889 mol HCO3Moles of Initial NaOH: Volume NaOH * M NaOH = initial moles NaOH 0.05 L NaOH * 0.09472 M NaOH = 0.004736 mol NaOH Moles NaOH reacted with HCl: Moles NaOH reacted with HCO3-: Initial moles NaOH – reacted moles NaOH = moles HCO30.004736 mol NaOH – 0.003099 mol NaOH = 0.001637 mol HCO3Moles of CO32-: Moles HCO3- - reacted moles HCO3- = moles CO320.002889 mol HCO3- - 0.001637 mol HCO3- = 0.001252 mol CO32Wt% of HCO3-: % HCO3- Wt% of CO32-: % CO32Finding an average: X1+X2+…+Xn / n 7.82+9.84+9.77 / 3 = 9.14 wt% Standard Deviation: ∑(xi − x̅)2 √ n−1 (3.33−4.02)2 +(4.92−4.02)2 +(3.81−4.02)2 √ 2 = 0.815 Conclusion: The objective of this lab was to determine the amount of a carbonate and bicarbonate species. Using the titration technique, we were able to determine the amount of carbonate as 9.14% and bicarbonate as 4.02%. As used in this lab, a primary standard is a reagent that is pure, can be weighed and used directly. The primary standard used in lab was the KHP. A secondary standard is one that is usually prepared in the lab and standardized against the primary standard. The secondary standard used in lab was the sodium hydroxide. An indirect titration is the same as a back titration. It involves a reagent being added to an analyte. The excess reagent remaining after the reaction with the analyte is determined by titration. The unknown was the indirect titration. A titrant is something added to the analyte until the equivalence point is reached. An indirect titration was more useful than a direct titration because it allowed us to find what percentage of carbonate and bicarbonate were in the unknown sample.