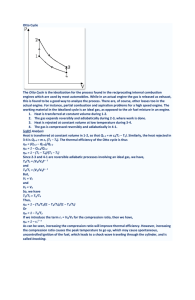
γ -1 - SNS Courseware
advertisement
Gas Power Cycles
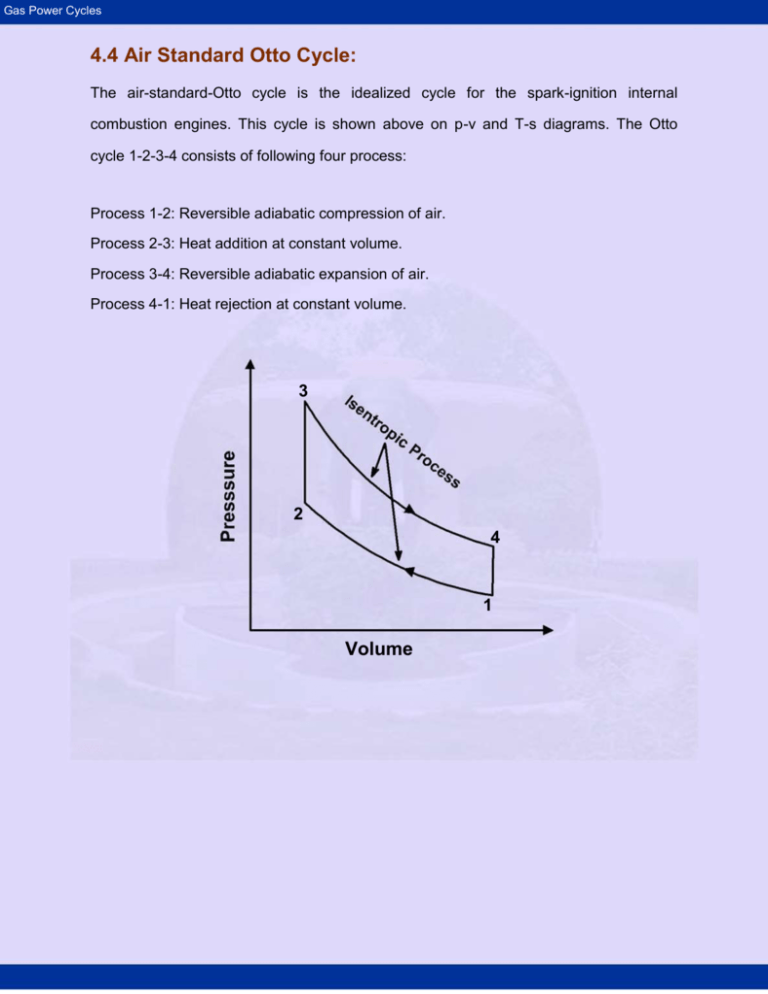
4.4 Air Standard Otto Cycle:
The air-standard-Otto cycle is the idealized cycle for the spark-ignition internal
combustion engines. This cycle is shown above on p-v and T-s diagrams. The Otto
cycle 1-2-3-4 consists of following four process:
Process 1-2: Reversible adiabatic compression of air.
Process 2-3: Heat addition at constant volume.
Process 3-4: Reversible adiabatic expansion of air.
Process 4-1: Heat rejection at constant volume.
3
2
4
1
Volume
Gas Power Cycles
3
2
4
1
Entropy
Fig.4.4. Otto cycle on p-v and T-s diagrams
Air Standard Efficiency:
ηth =
Net workdone
Net heat added
Since processes 1-2 and 3-4 are adiabatic processes, the heat transfer during the cycle
takes place only during processes 2-3 and 4-1 respectively. Therefore, thermal
efficiency can be written as,
ηth = Heat added - Heat rejected
Heat added
Consider ‘m’ kg of working fluid,
(T - T )
Heat Rejected = mC ( T - T )
Heat added = mC
V
3
V
ηth =
mC V
(T
- T ) - mC
32
V
(T
mC ( T - T )
V
32
4
2
4
-T
1
)
1
T -T
4
1
=1- T -T
3
2
Gas Power Cycles
For the reversible adiabatic processes 3-4 and 1-2, we can write,
T
T
=
4
v 3γ - 1
v
3
T
T
and
4
Vγ-1
V
=
1
2
2
1
v2 = v3 and v4 = v1
T
T
4
=
3
T
T
1
2
=
T -T
T -T
4
1
3
2
T
η th =
The ratio
1 -
1
T
V1
=
= 1-
V
V
γ−1
2
1
V2
γ-1
V1
2
is called as compression ratio, r.
V2
ηth = 1 - 1 γ 1
r
From the above equation, it can be observed that the efficiency of the Otto cycle is
mainly the function of compression ratio for the given ratio of C p and Cv. If we plot the
variations of the thermal efficiency with increase in compression ratio for different
gases, the curves are obtained as shown in Fig.4.4.1. Beyond certain values of
compression ratios, the increase in the thermal efficiency is very small, because the
curve tends to be asymptotic. However, practically the compression ratio of petrol
engines is restricted to maximum of 9 or 10 due to the phenomenon of knocking at high
compression ratios.
Gas Power Cycles
γ=1.67
γ=1.40
γ=1.30
Compression ratio,r
Effect of CR and γ on efficiency for Otto cycle.
Fig.4.4.1. Variation of thermal efficiency with compression ratio
Mean Effective Pressure:
Generally, it is defined as the ratio of the net workdone to the displacement volume of
the piston.
Let us consider ‘m’ kg of working substance.
Net work done = m Cv {(T3 - T2 ) - (T4 - T1 )}
(V
Displacement Volume =
1
mR
T
1
=
)
-V
2
r-1
1
r
=
V1 1 -
r
P1
= m Cv (γ- 1)T1 r - 1
=
P1 r
since, R = Cv ( γ - 1)
Indian Institute of Technology Madras
Gas Power Cycles
mC
mep =
(T
v
)-
-T
3
(T
2
m C v (γ -1) T
1
p
r-1
(r)
T =T
2
Let,
(T
-T
3
2
γ-1
)-(T
)
-T
4
1
}
T1 r - 1
1
r = P3 = T3 = Pressure ratio
p
P
T
2
2
T = P3 T
P
3
So,
r
{
γ
Now,
r
1
-1
1
1
P1
=
)
-T
4
=rT
2
r rγ-1
=
p 2
4
3
1
2
γ -1
= r rγ-1 T
=T 1
T
(for V = C)
T
p
r
1 γ-1
1
p
r
p 1
Pr
mep =
1
( r - 1) ( γ
r
γ-1
- 1)
r
(p
=Pr
=rT
{(
r
- ( rp - 1)
rγ -1 - rγ-1
)
p
-1 - r
)
-1
(p
)
( γ - 1) ( r - 1)
1
mep = P r
1
(rγ-1 - 1)(rp
-1
( r - 1) ( γ
- 1)
)
}