Otto Cycle Derivation
advertisement

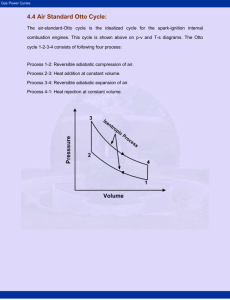
TEKNIK PERMESINAN KAPAL II (Minggu – 3) LS 1329 ( 3 SKS) Jurusan Teknik Sistem Perkapalan ITS Surabaya Gas Cycles Carnot Cycle T2 Heat Q 2 3 Work W T1 1 s1 4 s2 1-2 - ADIABATIC COMPRESSION (ISENTROPIC) 2-3 - HEAT ADDITION (ISOTHERMAL) 3-4 - ADIABATIC EXPANSION (ISENTROPIC) 4-1 - WORK (ISOTHERMAL) Carnot Cycle Carnot cycle is the most efficient cycle that can be executed between a heat source and a heat sink. T1 1T2 However, isothermal heat transfer is difficult to obtain in reality--requires large heat exchangers and a lot of time. Carnot Cycle Therefore, the very important (reversible) Carnot cycle, composed of two reversible isothermal processes and two reversible adiabatic processes, is never realized as a practical matter. Its real value is as a standard of comparison for all other cycles. Gas cycles have many engineering applications Internal combustion engine Otto cycle Diesel cycle Gas turbines Brayton cycle Refrigeration Reversed Brayton cycle Some nomenclature before starting internal combustion engine cycles More terminology Terminology Bore = d Stroke = s d 2 Displacement volume =DV = s 4 Clearance volume = CV Compression ratio = r VBDC DV CV r CV VTDC Mean Effective Pressure Mean Effective Pressure (MEP) is a fictitious pressure, such that if it acted on the piston during the entire power stroke, it would produce the same amount of net work. Wnet MEP Vmax Vmin The net work output of a cycle is equivalent to the product of the mean effect pressure and the displacement volume Real Otto cycle Real and Idealized Cycle Otto Cycle P-V & T-s Diagrams Pressure-Volume Temperature-Entropy Otto Cycle Derivation Thermal Efficiency: QH - QL QL =1th = QH QH For a constant volume heat addition (and rejection) process; Qin = m C v T Assuming QRej = m C v T constant specific heat: T4 T 1 - 1 m Cv ( T 4 - T 1 ) T = 1- 1 th = 1 m Cv ( T 3 - T 2 ) T3 T 2 - 1 T2 Otto Cycle Derivation For an isentropic compression (and expansion) process: -1 -1 T 2 = V 1 = V 4 = T 3 T1 V 2 T4 V 3 where: γ = Cp/Cv Then, by transposing, T3 = T4 T2 T1 Leading to T 1 th = 1 T2 Differences between Otto and Carnot cycles T 2 33 2 44 1 3 s Otto Cycle Derivation The compression ratio (rv) is a volume ratio and is equal to the expansion ratio in an otto cycle engine. Compression Ratio rv = V1 V4 = V2 V3 where Compression ratio is defined as Total volume v s + vcc = rv = Clearance volume vcc rv = vs +1 vcc Otto Cycle Derivation Then by substitution, 1- T 1 = V 2 = ( 1- rv ) T2 V1 The air standard thermal efficiency of the Otto cycle then becomes: 1 th = 1 - ( r v ) = 1 -1 ( rv ) 1- Otto Cycle Derivation Summarizing th = QH - QL Q =1- L QH QH Q = m C v T where T4 T 1 - 1 T th = 1 - 1 and T3 T 2 - 1 T2 T3 = T4 T2 T1 then T1 th 1 T2 1- Isentropic behavior T 1 = V 2 = ( 1- rv ) T2 V1 1 th = 1 - ( r v ) = 1 ( r v ) -1 1- Otto Cycle Derivation Heat addition (Q) is accomplished through fuel combustion Q = Lower Heat Value (LHV) BTU/lb, kJ/kg Qin F Q fuel cycle = ma A also Qin = m C v T Effect of compression ratio on Otto cycle efficiency Sample Problem – 1 The air at the beginning of the compression stroke of an air-standard Otto cycle is at 95 kPa and 22C and the cylinder volume is 5600 cm3. The compression ratio is 9 and 8.6 kJ are added during the heat addition process. Calculate: (a) the temperature and pressure after the compression and heat addition process (b) the thermal efficiency of the cycle Use cold air cycle assumptions. Draw cycle and label points 3 r = V1 /V2 = V4 /V3 = 9 P Q23 = 8.6 kJ 2 4 T1 = 295 K 1 P = 95 kPa 1 v Carry through with solution Calculate mass of air: P1V1 m 6.29 x 10-3 kg RT1 Compression occurs from 1 to 2: V1 T2 T1 V2 k 1 isentropiccompression T2 22 273K 9 1.41 T2 705.6 K But we need T3! Get T3 with first law: Q23 mcv T3 T2 Solve for T3: q 8.6 kJ 6.29x103 kg 705.6K T3 T2 cv 0.855 kJ kg T3 2304.7K Thermal Efficiency 1 1 r k 1 1 0.585 1 1.41 9 Sample Problem – 2 3 Solution P 2 4 1 v Diesel Cycle P-V & T-s Diagrams Sample Problem – 3 Gasoline vs. Diesel Engine