Defense Health Program Neurotoxin Exposure Treatment
advertisement

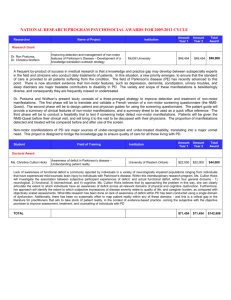
Defense Health Program Neurotoxin Exposure Treatment Parkinson's Research Program Funding Opportunities for Fiscal Year 2015 The Fiscal Year 2015 (FY15) Defense Appropriations Act provides $16 million (M) to the Department of Defense Neurotoxin Exposure Treatment Parkinson's Research (NETPR) Program to provide support for research of exceptional scientific merit leading to an understanding of the cause, prevention, and treatment of the loss of dopaminergic neurons in the Substantia nigra that result in Parkinson's disease (PD). Applications to the FY15 NETPR Program will be solicited by the U.S. Army Medical Research Acquisition Activity (USAMRAA). The managing agent for the anticipated Program Announcements/Funding Opportunities is the Congressionally Directed Medical Research Programs (CDMRP). http://cdmrp.army.mil/funding/netpr.shtml The mission of the NETPR Program is to: * Identify surrogate markers of PD, * Correlate distinctive clinical features with specific clusters of these markers, and * Develop interventions in bio-molecular pathways that link markers and expressed clinical features in order to prevent or halt progression of the disease or improve the quality of life for PD patients. Depression Research Award - Letter of intent due December 2, 2015 * Independent investigators at or above the level of Associate Professor (or equivalent). * Supports research efforts that will make significant advancements in the understanding of serotonergic and non-serotonergic molecular pathways identified with depression and associated biologic phenomena that link the pathways to other risk factors in the development and progression of PD. * Research supported by this award should lead to the development of new treatments or improvement of current treatments for depression, specifically in individuals with or at risk for PD. * Preliminary data are required. * This funding opportunity is specifically focused on the NETPR programs Areas of Emphasis on depression as a risk factor and/or co-morbidity in PD. * Innovative applications not focused on the Areas of Emphasis noted above are acceptable if specifically related to development or progression of depression as a non-motor manifestation of PD and/or is clearly linked to depression as a risk factor for initiation or progression of PD. * Submission of a Letter of Intent (LOI) is required prior to full application submission. * Clinical trials are not allowed. * The direct costs allowed and periods of performance are shown below. * Anticipation is that budgets will not exceed $1,500,000 in direct costs. * Period of performance not to exceed 3 years. Career Progression Award - Letter of Intent due December 2, 2015 * Independent investigators at or below the level of Assistant Professor (or equivalent). * Never received an R01 grant from the National Institutes of Health (NIH). * Supports independent, early-career investigators who have innovative, high-impact ideas or new technologies applicable to PD research and/or patient care. * Provides funding and experience necessary for productive career progression at the forefront of research specifically on non-motor aspects of PD. * Preliminary data are required. * This funding opportunity is specifically focused on the NETPR programs Areas of Emphasis for non-motor manifestations in PD (e.g., cognitive impairment, depression autonomic dysfunction, and sleep disturbance). * Innovative applications not focused on the Areas of Emphasis noted above are acceptable, provided the application addresses the development of progression of one or more non-motor manifestations of PD and provides a strong rationale for relevance to the mission of the NETPR program. * Submission of a Letter of Intent is required prior to full application submission. * Clinical trials are not allowed. * The direct costs allowed and periods of performance are shown below. * Anticipation is that budgets will not exceed $250,000 in direct costs. * Period of performance not to exceed 2 years. A pre-application is required and must be submitted through the electronic Biomedical Research Application Portal (eBRAP) at <https://eBRAP.org> https://eBRAP.org prior to the pre-application deadline. All applications must conform to the final Program Announcements and General Application Instructions that will be available for electronic downloading from the Grants.gov website. The application package containing the required forms for each award mechanism will also be found on Grants.gov.