video notes
advertisement
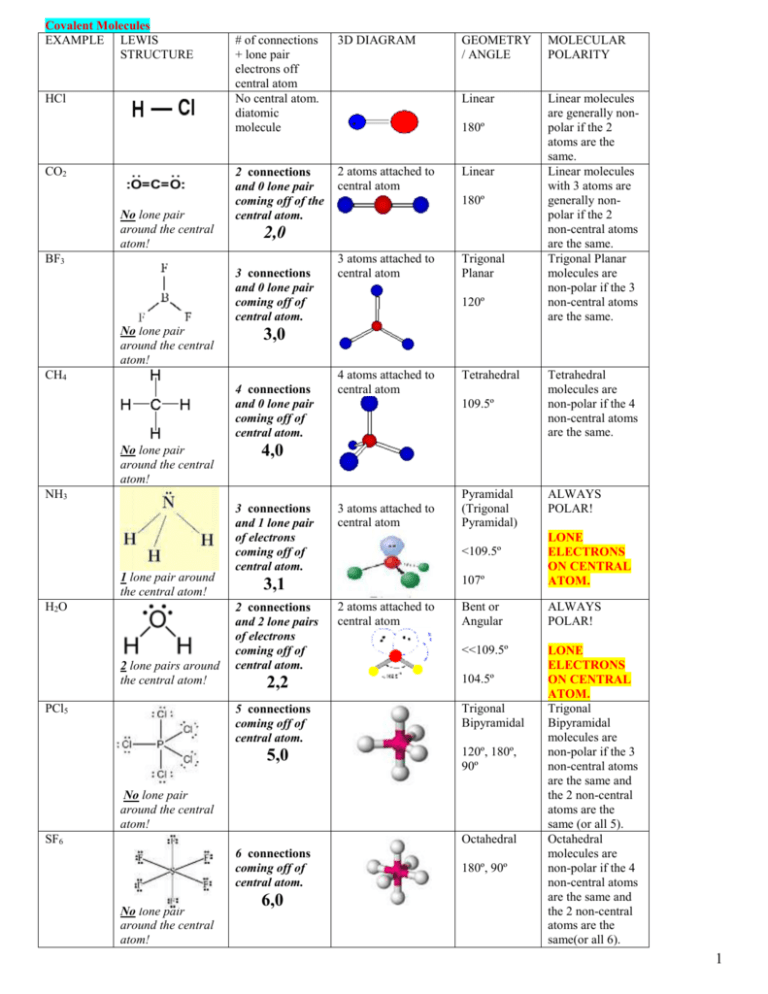
Covalent Molecules EXAMPLE LEWIS STRUCTURE HCl CO2 No lone pair around the central atom! # of connections + lone pair electrons off central atom No central atom. diatomic molecule 3D DIAGRAM 2 connections and 0 lone pair coming off of the central atom. 2 atoms attached to central atom 3 connections and 0 lone pair coming off of central atom. 4 connections and 0 lone pair coming off of central atom. H2O 2 lone pairs around the central atom! PCl5 Linear molecules are generally nonpolar if the 2 atoms are the same. Linear molecules with 3 atoms are generally nonpolar if the 2 non-central atoms are the same. Trigonal Planar molecules are non-polar if the 3 non-central atoms are the same. 180º Linear 180º 3 atoms attached to central atom Trigonal Planar 120º 4 atoms attached to central atom Tetrahedral 109.5º 3 connections and 1 lone pair of electrons coming off of central atom. 3 atoms attached to central atom 2 connections and 2 lone pairs of electrons coming off of central atom. 2,2 5,0 Pyramidal (Trigonal Pyramidal) 2 atoms attached to central atom 107º Bent or Angular ALWAYS POLAR! <<109.5º LONE ELECTRONS ON CENTRAL ATOM. Trigonal Bipyramidal molecules are non-polar if the 3 non-central atoms are the same and the 2 non-central atoms are the same (or all 5). Octahedral molecules are non-polar if the 4 non-central atoms are the same and the 2 non-central atoms are the same(or all 6). 104.5º Trigonal Bipyramidal 120º, 180º, 90º No lone pair around the central atom! SF6 Octahedral 6 connections coming off of central atom. 6,0 ALWAYS POLAR! LONE ELECTRONS ON CENTRAL ATOM. <109.5º 3,1 5 connections coming off of central atom. No lone pair around the central atom! Tetrahedral molecules are non-polar if the 4 non-central atoms are the same. 4,0 NH3 1 lone pair around the central atom! Linear 3,0 CH4 No lone pair around the central atom! MOLECULAR POLARITY 2,0 BF3 No lone pair around the central atom! GEOMETRY / ANGLE 180º, 90º 1 First: Review Ionic Bonding TYPES OF BONDING AND PROPERTIES http://youtu.be/Bjf9gMDP47s (even though it says it is metallic bonding, the video actually begins with ionic bonding. Since metallic bonding is next in the packet, watch the entire thing.) 1. IONIC BONDING (Metal + Nonmetal) Metal + monoatomic ion Metal + polyatomic ion Cation + anion A) In ionic bonding, electrons are ( transferred / shared) to attain a noble gas configuration. This process turns atoms into ___________. DEPICTING IONIC BONDING USING LEWIS STRUCTURES: HTTP://YOUTU.BE/K--NQD_MU7O METAL + NON-METAL Compounds Before Draw Lewis dot structures for the atoms found in the compound and use arrows to show the transfer of electron(s) After Use brackets around each ion formed and show the dots ONLY for the NON-METAL. The metal should have no dots in the “after” picture. Calcium chloride (Criss-cross to determine the formula) __________________ Aluminum Fluoride (Criss-cross to determine the formula) __________________ B) As a solid, the ions are in (random / fixed) positions. This makes ionic solids very stable, immobile and they do not_______________ electricity. Ionic solids are brittle. Ionic substances can ___________electricity when dissolved because ions are free to ___________. 2 C) It is relatively easy to dissolve most ionic solids in water because water is a very polar molecule. It is VERY difficult to melt or boil an ionic solid. In order to melt or boil an ionic crystal, you must break multiple ionic bonds. That is very difficult. Have you ever seen salt melt? This is why we can cook with salt and the salt doesn’t burn. Sugar is different. Sugar melts easily and burns easily. Do you think sugar is an ionic substance?________ PROPERTIES OF IONIC COMPOUNDS: http://youtu.be/6fam0LZV-8U 1._________________________________________________________________________ 2._________________________________________________________________________ 3._________________________________________________________________________ 4._________________________________________________________________________ 5. _________________________________________________________________________ Ex: 2. METALLIC BONDING: http://youtu.be/Bjf9gMDP47s Ex: Metals only! Metals often form lattices in the solid state Similar to ionic crystal lattices The metal atoms contribute their valence electrons to a “sea of electrons” Delocalized electrons - “sea” of mobile electrons The metallic bond is a bond between valence electrons and positively charged metal ions! PROPERTIES OF METALS: 1. Solid at room temperature (except Hg). 2. Luster (shine). 3. Malleable. 4. Ductile. 5. Conducts heat or electricity. Positive metal ions in fixed positions Mobile Valence electrons All because of mobile valence electrons! 3 The rest of this packet will focus on Covalent Bonding!!!! 3. COVALENT BONDING http://youtu.be/-v8XRaVBbDI (video is about drawing but watch it since drawing comes soon in this packet) https://youtu.be/MlgKp4FUV6I In covalent bonding, electrons are ( transferred / shared) to attain a noble gas configuration. non-metals ONLY! a. Regular COVALENT MOLECULES: COVALENT MOLECULES : NON-POLAR VS. POLAR MOLECULES 1. Determining if a molecule is polar: http://www.youtube.com/watch?v=OrLBWWZKmwY 2. polar and non-polar bonds. At end, it shows polar and non-polar molecules - http://youtu.be/un93VJxUfPA 3. http://www.brightstorm.com/science/chemistry/chemical-bonds/covalent-bonds/ 4. http://youtu.be/S8QsLUO_tgQ See my web page to copy definitions and properties below: A) NONPOLAR COVALENT MOLECULE: Definition: Properties: B) POLAR COVALENT MOLECULE: Definition: Properties: TRY: Label the substances below as: metallic, ionic, network covalent, molecular (non-polar) or molecular (polar). a. dissolves in water, does not conduct electricity as a solid, but does when dissolved in water_______________ b. dissolves in acetone, low boiling point__________________________ c. shiny, conducts electricity as a solid________________________ d. gas at room temperature______________________________ e. NH3__________________________________ f. NaBr_________________________________ g. CO2__________________________________ 4 Almost always Cl-Cl H-H O=O called a dipole. HF Depending on the symmetry. If the molecule is symmetrical, then the polarities cancel out!! Non-polar due to symmetry 4. Symmetrical molecules are non-polar. The drawings of these molecules are structural formulas. What can you see with a structural formula? 1. __types of a __ __ __ __ in the molecule. 2. __which atoms b __ __ __ to which other atoms 3.__arrangement in space____________________ 5 http://youtu.be/uYtwU0uRK7o 1. ENTIRE MOLECULES CAN BE POLAR OR NON-POLAR. Based on _______________________________________________ _______________________________________________ https://www.youtube.com/watch?v=02Q352-Y7iU 2. INDIVIDUAL COVALENT BONDS CAN BE POLAR OR NON-POLAR Based on differences in __________________________________ (subtract the values) Electronegativity Difference Bond Type 0.0 < difference ≤ 0.4 nonpolar covalent (equal sharing) 0.4 < difference < 1.67 polar covalent (unequal sharing) Difference ≥1.67 Ionic (transfer) 1. Use the chart above to classify the following bonds as either non-polar covalent, polar covalent or ionic. a. Si-P_________________________ b. H-O_________________________ c. Na-S_________________________ d. C-H_________________________ 2. Which bond is the most polar? Least polar? a. C-C b. C-Br c. C-F 6 DRAWING LEWIS STRUCTURES FOR COVALENT MOLECULES: https://youtu.be/G7crWDN9Q1c http://youtu.be/Ntwj-Qh12CQ 1. Count the total number of valence electrons (Group/Column #) – Total valence electrons 2. Draw stick figure. Choose a central atom. This is normally the first atom unless the 1st is hydrogen. Then choose 2nd atom. WHEN IN DOUBT, CARBON IS ALWAYS A CENTRAL ATOM! Arrange other atoms around central atom. (There may be more than one central atom) and place a BOND (A LINE) between adjacent atoms to represent SHARED ELECTRONS. Subtract two electrons for each bond drawn from the total. After subtraction - the # of electrons that you “HAVE”. 3. Count how many electrons each atom “NEEDS” to be stable. (H – 2, most others – 8) 4. *a. If NEED = HAVE, draw in the electrons as dots. *b. If NEED > HAVE, draw in 1 multiple bond for each difference of 2 between your NEED and HAVE BUT, NEVER GIVE A MULTIPLE BOND TO BORON (B), HALOGENS (COLUMN 7) or HYDROGEN (H)! c. If NEED < HAVE, you may give any extra to the central atom. 7. Draw in the rest of the dots (e-) to complete all octets (except H, He, Be, and B). Carbon will almost never have dots (electrons) - only bonds (exception) HINTS: C always in the center, always bonds 4x H, halogens never in center, always bond 1x O unless an ion, bonds 2x N usually bonds 3x HONC - 1234 Example: SiBr4 1. Si 4 valence electrons to offer Br 4(7) = 28 valence electrons to offer 4 + 28 = 32 2. Stick figure. Central atom Si 3. Subtract 8 electrons (4 bonds) from the total # of electrons from #1. This is your “Have” 32-8 = 24 have 4. Calculate your “need”. (Si is fine – it has 8 e around it but each Br needs 6 more so need is 24e and we have 24 so add the (dots) electrons. Final picture 7 http://youtu.be/sceYMpBrNNo H2O O2 PF3 6+6=12 draw stick figure O—O 12-2=10(have) CH4 Each O needs 6 so need 12. Difference of 2 between need and have so draw 1 multiple bond and then give each element the dots they need for an octet N2 BH3 (Watch it – exception!) central – B (3,0) Planar 120°, Non-Polar Don’t add another Bond even if need/ Have tells you to. CO2 CH2O HCN C2H6 C2H4 C2F2 8 SO4-2 ClO3-1 NH4+1 VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR) IS BASED ON: 1) Shared and unshared pairs of electrons repel each other yet an unshared pair of electrons repels more strongly than a shared pair 2) For the purpose of this model, a double or triple bond is considered equivalent to a single bond 3) The shape of a molecule or ion is the result of the shared and unshared pair of electrons being placed as far from each other as possible To apply VSEPR, we look at the central atom and count the # of shared and unshared pair of electron associated with it and this theory is based on placing electrons as far away from each other to minimize repulsions. See chart on p1 of packet Exceptions to the Octet Rule Rules: Second row elements C, N, O, F should always be assumed to follow the octet rule Second row elements Be and B often have fewer than eight electrons and are called electron-deficient. They are very reactive. Second row elements never exceed the octet rule since s & p can only have 8 eThird row and heavier elements often satisfy the octet rule but can exceed the octet rule by using their empty valence d orbitals If electrons remain, they should be placed on the atom that has a d orbital available and preferably the central atom. Ex. PCl5, I3-, ClF3, XeF4, BeCl2, ICl4- 9 Triple Bonds are (stronger / weaker ) and (shorter/ longer ) than double bonds. They are made of 1_________ and 2 ____ bonds. Double Bonds are (stronger / weaker ) and (shorter/ longer ) than single bonds. They are made of 1_________ and 1 ____ bonds. b. Special type of covalent bonding - NETWORK COVALENT BONDING: atoms are covalently bonded with each other WITHOUT ever forming separate molecules the bonds extend throughout the entire solid like one giant molecule THESE ARE THE STRONGEST COMPOUNDS!!!! PROPERTIES OF NETWORK COVALENT BONDING: http://youtu.be/PU9rzTjLyb4 (only watch until 4 min) http://youtu.be/fsc7_JbxDuY - understand why these substances have the highest melting points and boiling points of any substance on earth. Ex: 10 BONDING PRACTICE Lewis Structure Geometry (shape) Bond Angle Polarity of Bond Use electronegativity chart Polarity of Molecule OCl2 C2Br2 SiCl4 AsCl3 CH3F Si2Br4 SF2 CH2Cl2 11 Lewis Structure Geometry Bond Angle Polarity of Bond Polarity of Molecule SiH2Se SO3-2 C2HBr CBr4 SO4-2 12 VSEPR DIAGRAMS AND GEOMETRY 1. Complete the chart below. SCRATCH WORK STRUCTURAL, LEWIS OR VSEPR DIAGRAM NAME OF SHAPE BOND ANGLE IS THE MOLECULE POLAR? MP/BP? DISSOLVES IN? (YES OR NO?) (dispersion or dipole?) Cl2 O2 HBr CH2S 13 14 DIAGRAM (scratch work) STRUCTURAL, LEWIS OR VSEPR DIAGRAM NAME OF SHAPE BOND ANGLE IS THE MOLECULE POLAR? MP/BP DISSOLVES IN? (YES OR NO?) (dispersion or dipole?) CF4 PH3 H2S CS2 15 DIAGRAM (scratch work) STRUCTURAL, LEWIS OR VSEPR DIAGRAM NAME OF SHAPE BOND ANGLE IS THE MOLECULE POLAR? MP/BP DISSOLVES IN? (YES OR NO?) (dispersion or dipole?) CF2Cl2 PF3 OH-1 CO3-2 16 COORDINATE COVALENT BONDING Example: O3 PRACTICE WITH COORDINATE COVALENT EXAMPLES: Formula Lewis Structure Molecular Shape (diagram) Name of Shape Bond Angle 1. NO3-1 2. SO2 3. SO3-2 17 Work Space NO3-1 Work Space SO2 Work Space Work Space SO4-2 18 19 HOMEWORK #1: INTERMOLECULAR FORCES 1. Label the substances below as: metallic, ionic, network covalent, molecular (nonpolar (dispersion)), molecular (polar (dipole)) or molecular (hydrogen bonding) a. melts at 800°C, conducts electricity as a solid, does not dissolve in water __________________ b. strong odor, dissolves in gasoline, melts easily when heated __________________ c. crystalline solid, dissolves in water, solution conducts electricity __________________ d. crystalline solid, does not dissolve in water or acetone, very high melting point _______________ e. K2SO4 __________________ f. CH4 __________________ g. H2O __________________ 2. Fill in the missing boxes in the chart below. The types of solid used are: Molecular (nonpolar), Molecular (polar); Ionic, Network Covalent, Metallic Appearance Time to Melt? Dissolves in? (water, alcohol, acetone) Conductivity? 1 crystalline solid > 10 minutes* water (*would not melt in our lab) 2 Shiny solid > 10 minutes* (*would not melt in our lab) 4 minutes 3. crystalline solid flaky solid 30 seconds none > 10 minutes* did not dissolve (*would not melt in our lab) none Crystalline solid Type? As solid; liquid not tested water, alcohol 4. 5. 20 HOMEWORK #2: DRAWING LEWIS 1. Draw each molecule below with the correct bonding. Follow the steps below: a. Position atoms as symmetrically as possible. b. Count to make sure all atoms are up to 8 electrons, except hydrogen (2) or boron (6). a. H2O b. HOBr c. H2CS d. HCP e. PH3 f. HCN g. CS2 h. BF3 i. CCl4 2. Draw a Lewis dot diagram for each ionic compound below: a. RbCl b. AlF3 21 HOMEWORK #3: LEWIS DIAGRAMS SCRATCH WORK STRUCTURAL, LEWIS OR VSEPR DIAGRAM NAME OF SHAPE BOND ANGLE IS THE MOLECULE POLAR? (YES OR NO?) (dispersion or dipole?) H2Se AsF3 BF3 **B is an exception, it only gets 6 N2 SiCl4 2. Complete the chart below: Formula Lewis Structure Molecular Shape (diagram) Name of Shape Bond Angle Polar? Dipole or Dispersion? 1. PBr3 22 MP/ 2. C2Cl2 3. SiCl4 4. O2 5. PO43- 23





