Partial Differential Equations in Two or More Dimensions
advertisement

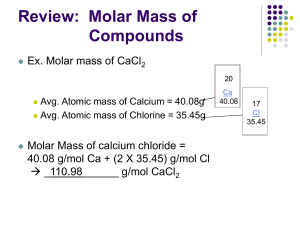
Appendix A Previous Exams CHE31301 MASS TRANSFER QUIZ #1 Spring 2011 I. A vertical pipe (25-mm ID), 2 m long, and closed at the bottom contains a 25-cm-deep layer of water at the bottom. The temperature and pressure of the surrounding gas (pure nitrogen) are 21oC and 760 mm Hg respectively. The vapor pressure of water at 21oC is 19 mm Hg. The nitrogen in contact with the open end of the pipe is dry. 1. The mass fraction of water vapor at the water surface is 0.016216 2. A. The mole fraction of water vapor at 1.0 m from the open end is independent of time. B. The mole fraction of water vapor at the gas-liquid interface is independent of time. a. A and B are true b. Only A is true c. Only B is true d. A and B are false 3. A. If the surrounding nitrogen is saturated with water vapor, there will be no (net) evaporation. B. It will take longer time for the water level to drop if the diameter is 50-mm ID, everything else remains the same. a. A and B are true b. Only A is true c. Only B is true d. A and B are false 4. A. The molar average velocity is constant within the pipe. B. vA (A = water vapor) is constant within the pipe. a. A and B are true b. Only A is true c. Only B is true 5. If the mole fraction of H2O is 0.1 then the mass fraction of H2O is A-1 d. A and B are false 0.066667 II. Pulverized coal pellets, which may be approximated as carbon spheres of radius ro = 1 mm, are burned in a air (21% O2) at 1500 K and 1 atm. Oxygen is transferred to the particle surface by diffusion, where it is consumed in the reaction C + O2 CO2. The diffusivity DA for O2 is 1.810-4 m2/s. Assuming the surface reaction rate to be infinite and neglecting change in ro, a) (5 pts) Determine the steady-state O2 molar consumption rate in kmol/s. (Show all your work) Gas constant Rg = 0.08206 m3atm/(kmolK) 3.86×10-9 kmol/s II. Pulverized coal pellets, which may be approximated as carbon spheres of radius ro = 1 mm, are burned in a air (21% O2) at 1500oK and 1 atm. Oxygen is transferred to the particle surface by diffusion, where it is consumed in the reaction C + O2 CO2. The diffusivity DA for O2 is 1.810-4 m2/s. Assuming the surface reaction rate to be infinite and neglecting change in ro, b) (5 pts) Determine the mole fraction of O2 at r = 3 mm. (Show all your work) 0.14 A-2 CHE31301 MASS TRANSFER QUIZ #2 Spring 2011 Note: Your answers must be correct to 3 significant figures and have the appropriate units. I. A biofilm consists of living cells immobilized in a gelatinous matrix. A toxic organic solute (species A) diffuses into the biofilm and is degraded to harmless products by the cells within the biofilm. We want to treat 0.2 m3 per hour of wastewater containing 0.14 mole/m3 of the toxic substance phenol using a system consisting of biofilms on rotating disk as shown below. Waste water feed stream biofilm CA0 CA(z) Inert solid surface CA0 biofilm Treated z=0 waste water Well-mixed contactor Determine the required surface area of the biofilm with 1 mm thickness to reduce the phenol concentration in the outlet stream to 0.025 mole/m3. The rate of disappearance of phenol (species A) within the biofilm is described by the following equation rA = − k1cA where k1 = 0.0024 s-1 The diffusivity of phenol in the biofilm at the process temperature of 25oC is 2.0×10-10 m2/s. Phenol is equally soluble in both water and the biofilm. 1) Determine the rate of phenol processed by the biofilms in mol/h: 0.0230 mol/h 2) The concentration of A in the biofilm is give by cA = B1sinh(mz) + B2cosh(mz), the numerical value of m is 3,464.1 m-1 A-3 For questions (3) and (4) use m = 3 mm-1, determine the numerical values with units for 3) B2 = 0.024876 mole/m3 For the next question use cA = 0.025 4) B1 = 0.025 mole/m3 cosh 3.5( z ) where z is in mm and cA is in mole/m3 cosh 3.5 5) Determine the molar flux of A at z = 0 1.75×10-8 mol/m2s 6) Determine the molar flux of A at z = 0.5 mm 2.95×10-9 mol/m2s II. In a hot combustion chamber, oxygen diffuses through a stagnant film of air with thickness L to the carbon surface where it reacts to make CO and CO2. The mole fraction of oxygen just outside the stagnant film (z = 0) is 0.21. The reaction may be assumed to be instantaneous. No reaction occurs in the gas film. The chamber is at 1 atm, 1000 K, and L = 10 cm. The diffusivity of oxygen at these conditions is 0.35 cm2/sec. The following reaction occurs at the carbon surface: 4C + 3O2 2CO + 2CO2 (7) Determine the molar flux of oxygen in mol/scm2 (8) Determine the mole fraction of oxygen at z = 4 cm A-4 8.6610-8 mol/cm2s 0.12429 III. membrane Figure III. Conceptual model for a bioartificial organ. Oxygen transport is of critical importance in the design of bioartificial organs. In one type of device, therapeutic cells such as the islets of Langerhans for the treatment of diabetes, are contained within a spherical membrane shown in Figure III. Let R be the distance from the center of the organ to the inside surface of the membrane, met be the tissue metabolic volumetric oxygen consumption rate (met is a constant, which is the rate of oxygen consumption per unit tissue volume), De be the diffusivity of oxygen within the tissue space, and be the void fraction within the tissue space. The concentration of oxygen at the inside surface of the membrane is CA0. 9) The oxygen concentration, CA, within the tissue space can be obtained by solving the following differential equation Ans: _________ A) De d 2 dC A r = met r 2 dr dr B) De d 2 dC A r = met r 2 dr dr C) De d 2 dC A r = met(1 ) (ANS.) r 2 dr dr D) None of the above. 10) The two boundary conditions required to solve the above equation are: r = R, CA = CA0 r = 0, A-5 dC A =0 dr CHE31301 MASS TRANSFER QUIZ #3 Spring 2011 Note: Your answers must be correct to 3 significant figures and have the appropriate units. I. XI. The concentration of A within the biofilm is given by k cosh[ m( L z )] , where m2 = 1 DA cosh( mL) -4 k1 = first order rate constant = 1.5x10 /s DA = diffusivity of A in the biofilm = 1.26x10-5 cm2/s L = biofilm thickness = 0.2 cm z = distance from the outer (solution) surface of the biofilm CAo = 0.4 mol/liter solution CA = CAo biofilm solid 1) If the mass transfer resistance in the solution is negligible and the concentration of A in the biofilm is related to the concentration of A in the solution (CA) by the equilibrium relation CA = 0.4 CA , determine CA CA = 1.0 mol/liter 2) If the surface area of the biofilm is As, the rate of substrate consumption within the biofilm is given by A) k1CAAsL B) CAomAsDAtanh(mL) (ANS.) C) k1CAoAsL D) None of the above. II. Consider a spherical organism of radius R within which respiration occurs at a uniform volumetric rate of rA = k1CA. That is, oxygen (species A) consumption is governed by a firstorder, homogeneous chemical reaction. A molar concentration of CA(R) = CA,0 is maintained at the surface of the organism, and an expression for the radial distribution of oxygen, CA(r), within the organism is given by CA(r) = CA,0 R sinh( r ) r sinh( R) Data: R = 0.16 mm, diffusion coefficient for oxygen transfer DAB = 10-8 m2/s, CA,0 = 610-5 kmol/m3, and k1 = 30 s-1. 3) The numerical value of is 5.48104 m-1 4) If = 5104 m-1, the concentration of oxygen at the center of the organism is kmol/m3 A-6 3.2210-7 5) If = 5.0104 m-1, molar flux of A at r = R is 2.62×10-8 kmol/m2 6) If = 5.0104 m-1, molar flux of A at r = 0 is _____0_____ III. A solute diffuses through a membrane that separates two compartments A and B that have different initial concentrations. The solute concentrations in the two compartments as a function of time, CA and CB are shown in Figure III-1. The volumes of the two compartments are VA and VB. (A) VA < VB (B) Solute diffuses from compartment B to A. a. A and B are true b. Only A is true 10 c. Only B is true d. A and B are false CA 5 CB 0 t Figure III-1. Concentration of solute as a function of time in compartments A and B. IV. Pulverized coal, which may be approximated as carbon spheres of radius R = 1 mm, is burned in a pure oxygen atmosphere at conditions such that carbon monoxide is produced. Oxygen is transferred to the particle surface by diffusion, where it is consumed in the reaction C+ 1 O2 CO 2 Let N”A be the molar flux of O2 and N”B be the molar flux of CO, we have a) N”A = 2N”B b) N”A = 2N”B c) N”A = 0.5N”B(ANS) d) N”A = N”B A-7 IV. The “drug patch” shown in the figure below releases a water-soluble epidermal growth factor (species A) to repair a specific region of wounded tissue on the human body. A slow release of the drug is critical for regulating the rate of tissue repair. The drug layer (pure solute A) rests on top of a diffusion barrier. The diffusion barrier is essentially a micro-porous polymer material consisting of tiny parallel pores filled with liquid water (species B). The diffusion barrier controls the rate of drug release. The thickness (L), pore size (dpore), and porosity of the diffusion layer determine the dosage rate of the drug to the tissue directly beneath it. The maximum solubility of the drug in water is 1 mole/m3 at 25oC. The total surface area of the patch is 4 cm2, but the cross-sectional area of the pores constitute only 35% of the surface area for flux. The effective diffusion coefficient of the drug in the diffusion barrier is 1.810-11 m2/s. Drug patch Drug reservoir Impermeable barrier Diffusion barrier (water-filled micropores) L Skin surface Infected body tissue (sink for drug) (9) If the thickness of the diffusion barrier (L) is 5 mm, determine the dosage rate in mole per second, assuming that the drug is instantaneously consumed once it exits the diffusion barrier and enter the body tissue. ____________ WA = 5.0410-13 mol/s (10) The diffusion coefficient of the drug in water is 1.4010-10 m2/s at 25oC. However, the human body is actually at 37oC, what is the drug diffusivity in water if the temperature is increased to 37oC? Variation of the drug diffusivity can be predicted by the Stoke Einstein equation RT D= ____________ 6aN A Data: Viscosity of water at 25oC is 0.9078 cP, viscosity of water at 37oC is 0.7074 cP. D = 1.86910-10 m2/s A-8 CHE31301 MASS TRANSFER QUIZ #4 Spring 2011 Note: Your answers must be correct to 3 significant figures and have the appropriate units. I. Consider the transport of glucose from capillary blood to exercising muscle tissue. As a basis consider 1 gram of tissue. The glucose consumption of the tissue is 0.015 mol/gs. Blood flow to the region is 0.01 cm3/gs. The arterial glucose concentration is 5 mol/cm3. The value of PmS based on capillary recruitment during exercise is 0.004 cm3/ gs. Using the CSTR model calculate: 1) the glucose concentration in the tissue space 1.5 mol/cm3 2) the glucose concentration in the exit blood. 3.5 mol/cm3 II. In a hot combustion chamber, oxygen diffuses through a stagnant film of air with thickness L to the carbon surface where it reacts to make CO and CO2. The mole fraction of oxygen at z = L is 0.21. The reaction may be assumed to be instantaneous. No reaction occurs in the gas film. The chamber is at 1.5 atm, 1000oK, and L = 0.1 m. The diffusivity of oxygen at these conditions is 0.35 cm2/sec. The following reaction occurs at the carbon surface (Gas constant = 0.08205 atmm3/kmoloK): 4C + 3O2 2CO + 2CO2 (3) a) NO2 = 2 NCO2 3 b) NO2 = 1.5 NCO2 c) NO2 = 2 NCO2 3 d) None of the above III. A mixture contains 35 mole % isobutane and 65 mole % isopentane is at 30 psia. The K values for these compounds can be obtained from ln K = A/T2 + B + C ln P where T is in oR and P is in psia Compound A B Isobutane -1,166,846 7.72668 Isopentane -1,481,583 7.58071 C -.92213 -.93159 4) The vapor mole fraction of isobutane at the bubble point of 542.3oR is 0.6524 5) The liquid mole fraction of isobutane at the dew point of 562.11oR is 0.1427 IV. (6) The flux of oxygen across a 75-mil-thick polypropylene film at 30oC is 3510-9 mol/m2.s per atmosphere of oxygen partial pressure difference. Determine the flux of oxygen across a 30mil-thick film where the left side is open to air at 1 atm with 21 mol % oxygen and the right side is in vacuum with no oxygen. 18.37510-9 mol/m2.s A-9 V. In an experimental study of the absorption of ammonia by water in a wetted-wall column, the value of KG was found to be 2.75×10-6 kmol/m2skPa. At one point in the column, the composition of the gas and liquid phases were 8.0 and 0.115 mole % NH3, respectively. The temperature was 300 K and the total pressure was 1 atm. Eighty percent of the total resistance to mass transfer was found to be in the gas phase. At 300 K, ammonia-water solution follow Henry’s law up to 5 mole % ammonia in the liquid, with m = 1.64 when the total pressure is 1 atm or 101.3 kPa. 7) Determine ky 3.48×10-4 kmol/m2s 8) Determine the ammonia absorption rate in kmol/m2s 2.18×10-5 kmol/m2s VI.) (9&10) Consider a glass tube with length L = 2 m where the end plates and fittings of the tube impermeable to hydrogen. The molar solubility ratio S of H2 (species A) in glass is 0.2 (mol/cm3) H2 in glass/(mol/cm3) H2 in gas. The inside and outside radius of the tube are ri and ro, respectively. DAB is the diffusivity of hydrogen in glass. cgi and cgo are the molar concentrations of H2 inside and outside the tube, respectively. (Gas constant = 82.06 atmcm3/molK). The diffusivity of H2 in glass at 300 K, is 0.4×10-8 cm2/s. The hydrogen pressure inside the tube is 2 atm at 300 K and the partial pressure outside the tube is zero. If ri = 1 cm and ro = 1.5 cm, determine the hydrogen leak rate in gmol/s. Show all your work! Scgi = 1.6210-5 mol/cm3 WA = 2.008310-10 mol/s A-10 CHE31301 MASS TRANSFER QUIZ #5 Spring 2011 Note: Your answers must be correct to 3 significant figures and have the appropriate units. I. Nitric oxide (NO) emissions from automobile exhaust can be reduced by using a catalytic converter, and at the catalytic surface: NO + CO → 0.5 N2 + CO2 The concentration of NO is reduced by passing the exhaust gases over the surface, and the rate of reduction at the catalyst is governed by a first order reaction of the form r"A(kmol/m2∙s) = k”1CA where k”1 = 0.08 m/s and A denotes NO Exhaust gas at T and P yA0 z=0 Catalytic surface z=L As a first order approximation it may be assume that NO reaches the surface by one-dimensional diffusion through a thin gas film of thickness L that adjoins the surface. Consider a situation for which the exhaust gas is at 750oK and 1.2 bars and the mole fraction of NO is yA0 = 0.25 if DA,mix = 10-4 m2/s and the film thickness is L = 1 mm. (1) The molar flux of A (NO) can be obtained by integrating the following expression cD AB dy A (1 0.5 y A ) dz (A) NA = (Ans.) (C) NA = cDAB cD AB dy A (1 0.5 y A ) dz (B) NA = dy A dz (D) None of the above (2) If the molar of A is given by NA = cDAB(yA0 yAL)/L determine yAL 0.139 II) A distillation column with 100 kmol/h feed of 60% A and 40% B produces a distillate product with xD = 0.95 and a bottom stream with xbot = 0.04 of the more volatile species A. CMO 2.5 x is valid and the equilibrium data is given by y = 1 1.5 x 3) For total reflux, determine (numerically) the composition (y) of the vapor stream entering the second equilibrium plate from the top. 0.7525 A-11 4) For a reflux ratio of 2, and q = 0.5, determine the liquid composition (x) of the feed point (the intersection of the q-line and the operating lines). x = 0.53 III. A mixture contains 35 mole % isobutane and 65 mole % isopentane is at 30 psia. The K values for these compounds can be obtained from ln K = A/T2 + B + C ln P where T is in oR and P is in psia Compound A B Isobutane -1,166,846 7.72668 Isopentane -1,481,583 7.58071 C -.92213 -.93159 5) The mixture is flash at 552.1 oR, 30 psia where V/F = 0.4, then the mole fraction of isobutane (iC4) in the liquid phase is A-12 0.2402 IV) Consider a distillation column with the McCabe-Thiele diagram given in Figure Q4-III. The column has a total condenser and a partial reboiler. Note: the points A, C, E, a, c, and e are on the equilibrium curve and the points B, D, F, b, d, and f are on the operating lines. Figure Q4-III McCabe-Thiele diagram for binary distillation. 6) A) The x coordinate of point D gives the mole fraction of the volatile species in the liquid stream entering equilibrium tray 4 from the top (with the top tray as tray 1). B) The y coordinate of point C gives the mole fraction of the volatile species in the vapor stream entering equilibrium tray 3 from the top. a. A and B are true. b. Only A is true (A) c. Only B is true d. A and B are false 7) A) The y coordinate of point d gives the mole fraction of the volatile species in the vapor stream entering equilibrium tray N-2 (with the bottom tray before the reboiler as tray N). B) The x coordinate of point d gives the mole fraction of the volatile species in the liquid stream leaving tray N-2. a. A and B are true (A). b. Only A is true c. Only B is true 8) A) The feed is introduced into tray 6 from the top. B) The feed is introduced at the optimum location in the column. a. A and B are true. b. Only A is true c. Only B is true (A) A-13 d. A and B are false d. A and B are false V, yD V) A liquid feed at the boiling point contains 5 mol % of species A and 95 mol % water and enters the top tray of a stripping tower shown in Figure Q4-V. Saturated steam is injected directly into liquid in the bottom of the tower. The overhead vapor which is withdrawn contains 98% of A in the feed. Assume equimolar overflow for this problem. Equilibrium data for mole fraction of A is given by y = 10x for x 0.08. (9) For an infinite number of theoretical steps, calculate the minimum moles of steam needed per mole of feed. F xF L x S (Steam) V y B, xB Figure Q4-V Stripping tower Minimum 0.098 mol steam/mol feed (10) Using 15 moles of steam per 100 moles of feed, calculate the mole fraction of A in the vapor 0.3267 A-14