Chemistry I Final Exam Study Guide - High School
advertisement

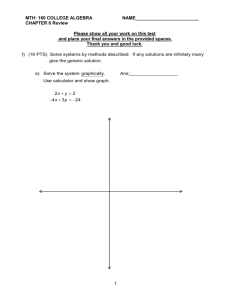
Chemistry I Final Exam Study Guide Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Which of the following is a prefix that makes the unit larger? a. kiloc. millib. decid. pico- ____ 2. A milliliter is the same volume as a a. cubic centimeter. b. millimeter. ____ ____ ____ c. centimeter. d. cubic meter. 3. A recorded measurement has two certain digits and one estimated digit. How many significant digits does the measurement have? a. none c. two b. one d. three 4. In multiplication and division of measured values, the measured value that determines the number of significant digits in the answer is the one that has the a. largest number of significant digits. c. largest number of decimal places. b. smallest number of significant digits. d. smallest number of significant zeros. 5. The percent error is equal to 100 percent multiplied by a. b. c. d. MASS OF SAMPLE 4078 Team 1 Team 2 Team 3 Team 4 Reading 1 42 g 41.04 g 31.33 g 42.34 g Reading 2 42.158 g 39.77 g 31.30 g 41.12 g Reading 3 42.07 g 43.15 g 31.36 g 41.21 g Average 42.1 g 41.32 g 31.33 g 41.55 g Accepted measure from issuing lab: 41.33 g Percent error 1.9% –0.02% –24.2% Figure 1-3 0.53% ____ 6. Why might the measurements from Team 1 in Figure 1-3 be thought to be from different ____ 7. ____ 8. ____ 9. ____ 10. ____ 11. instruments? a. Each measurement is taken to a different level of precision. b. The values of the readings are so far apart. c. The percent error is so large. d. The percent error is positive. Which team in Figure 1-3 is most accurate? a. team 1 c. team 3 b. team 2 d. team 4 Which team in Figure 1-3 is most precise? a. team 1 c. team 3 b. team 2 d. team 4 In Figure 1-3, how many significant figures should be recorded in Team 4's average? a. correct as written c. five b. four d. need more information In Figure 1-3, what is Team 2's average written in scientific notation? a. 41.32 g c. 4.1 1032 g 32 b. 41 10 g d. 4.132 101 g In Figure 1-3, which team data should be used in further calculations? a. team 2 c. team 4 b. team 3 d. none of the teams Completion Complete each statement. 12. ____________________ is the closeness of a measurement to the actual value being measured. 13. The three values 10.714 m, 12.821 m, and 13.646 m have the same number of ____________________. 14. A ____________________ description of a scientific law would use a mathematical equation. 15. Although a measurement of 6.13457902 cm is very ____________________, it may not be accurate. 16. A possible answer to a scientific problem is a(n)____________________. 17. When examining a mineral, hardness and color are two of the properties used for identification. Hardness and color are examples of ____________________ properties. 18. An apple turning brown after being cut is an example of a ____________________ change. 19. Some iron and sulfur are mixed together, then heated. When the result is cooled, the iron can no longer be separated from the sulfur with a magnet. The result of heating the mixture was the formation of a(n) ____________________. 20. A sample of matter can be poured from container to container. It takes the shape of its container but only takes up a certain volume. Based on this information, the sample is in the ____________________ state. 21. The particles of a substance in the ____________________ are able to slide past each other. 22. The two types of matter that are pure substances are ____________________ and ____________________. 23. An alloy such as a gold ring is an example of a(n) ____________________ mixture. 24. Gravel is an example of a(n) ____________________ mixture. 25. A solid that forms and separates from a liquid mixture is a(an) ____________________. 26. When two or more pure substances are blended together, the result is a(n) ____________________. 27. A ____________________ property describes how a substance acts when it reacts with other substances. 28. Flammability is a chemical property that tells whether a substance reacts in the presence of ____________________. 29. A(an) ____________________ has a definite volume and a definite shape. 30. ____________________ between the particles of a gas and the walls of the container cause pressure in a closed container of gas. 31. As gas particles bounce around and collide, they spread to ___________________________________. 32. A solid holds its shape because its structure is ____________________. 33. Any factor in an experiment that can change is referred to as a(n) ____________________. 34. The number 50.775 has ____________________ significant figures. 35. Elements with similar properties, listed in a single column on the periodic table, form what is called a _________________________. Matching Match the following terms with the correct definition. There is one extra term which will not match any of the definitions. a. brittleness b. elasticity c. hardness d. malleability e. density f. tensile strength ____ ____ ____ ____ ____ 36. 37. 38. 39. 40. A measure of how much pulling a material can stand before breaking A measure of a material's tendency to shatter upon impact A measure of a solid's resistance to scratching The measure of a solid's ability to be stretched and then returned to its original size A solid's ability to be pounded into thin sheets Match each part of the atom with its identity from the list below. ____ ____ ____ ____ ____ 41. 42. 43. 44. 45. energy level neutron proton electrons nucleus For the following element, match the letter with the type of information given. ____ ____ ____ ____ 46. 47. 48. 49. name of element atomic mass symbol atomic number Short Answer 50. For the nucleus shown below, do the following: A. Name the element. B. Give the mass number. C. Show the isotope notation. 51. How many energy levels would be completely filled by a neutral atom of fluorine? How many electrons would be left over? Essay PROBLEM SOLVING Use the skills you have developed in this chapter to solve each problem. 52. Each of four students separately makes four measurements of the mass of the same object. The readings are as follows: Student 1: 9.1 g, 9.2 g, 9.1 g, 9.1 g Student 2: 11 g, 9 g, 9 g, 11 g Student 3: 14.1 g, 11.0 g, 17.4 g, 18.8 g Student 4: 10.1 g, 9.9 g, 10.0 g, 10.0 g. The real value of the object's mass is 10.0 g. Evaluate each student's set of readings in terms of precision and accuracy. 53. Determine the number of significant digits in each of the following measured values: (a) 6.7090; (b) 0.0384; (c) 12,000; (d) 3400.; (e) 100.050. 54. Calculate the volume of a rectangular pan that is 27.0 cm long, 14.55 cm wide, and 9.3 cm high. (Remember to retain only significant digits in your final answer and to express your answer in the proper units.) 55. A student measures the mass of an object as 135.80 g. Calculate the percent error in the measurement, given that the accepted value for the mass is 137.23 g. 56. Find the measured mass of a sample if the accepted value is 20 grams and the percent error is 2 percent. 57. Calculate the density of an object that occupies 17.1 cm3 and has a mass of 39.26 g. Will that object float in water, given that the density of water is 1.00 g/cm3? Explain your answer. 58. Convert 73.0 seconds to weeks. Express your answer in scientific notation. 59. Calculate how many meters are in one mile (39.37 inches = 1 meter, 5286 feet = 1 mile). Express your answer in scientific notation. 60. Convert a height of 5 feet, 6 inches to meters. (2.54 cm = 1 in.) 61. Compare and contrast precision and accuracy. Chemistry I Final Exam Study Guide Answer Section MULTIPLE CHOICE 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: A A D B B A B C B D A PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 1 1 1 1 1 1 OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: 1A 1.d 1A 1.d 1A 1.g 1A 1.g 1A 1.h 1A 1.e 1A 1.f 1A 1.f 1A 1.g 1A 1.g 1A 1.h COMPLETION 12. ANS: Accuracy PTS: 1 DIF: L1 13. ANS: significant figures OBJ: 1.3.3 BLM: knowledge PTS: 1 14. ANS: quantitative DIF: L2 OBJ: 1.3.3 BLM: comprehension PTS: 1 15. ANS: precise DIF: 2 REF: 1 OBJ: 3 PTS: 1 16. ANS: hypothesis DIF: 1 REF: 3 OBJ: 3 PTS: 1 17. ANS: physical DIF: 1 REF: 2 OBJ: 1 PTS: 1 18. ANS: chemical DIF: I REF: 2 OBJ: 1 PTS: 1 19. ANS: compound DIF: II REF: 2 OBJ: 2 PTS: 1 20. ANS: liquid DIF: II REF: 2 OBJ: 2 PTS: 1 DIF: II REF: 2 OBJ: 3 21. ANS: liquid PTS: 1 DIF: I 22. ANS: elements, compounds REF: 2 OBJ: 3 PTS: 1 DIF: II 23. ANS: homogeneous REF: 2 OBJ: 5 PTS: 1 DIF: I 24. ANS: heterogeneous REF: 2 OBJ: 5 PTS: 1 25. ANS: precipitate DIF: II REF: 2 OBJ: 5 PTS: 1 BLM: knowledge 26. ANS: mixture DIF: L1 OBJ: 2.3.2 STA: SC-HS-1.1.8.C PTS: 1 27. ANS: chemical DIF: 1 REF: 1 OBJ: 4 PTS: 1 DIF: 1 STA: SC.HS.1.1.1| SC.HS.1.1.5 28. ANS: oxygen REF: 2 OBJ: 2 PTS: 1 DIF: 2 STA: SC.HS.1.1.1| SC.HS.1.1.5 29. ANS: solid REF: 2 OBJ: 2 PTS: 1 30. ANS: Collisions DIF: L1 OBJ: 3.1.1 BLM: knowledge PTS: 1 DIF: L1 31. ANS: fill all available space OBJ: 3.2.1 BLM: knowledge PTS: 1 STA: SC.HS.1.1.3 32. ANS: rigid DIF: 1 REF: 4 OBJ: 1 PTS: 1 STA: SC.HS.1.1.3 33. ANS: variable DIF: 1 REF: 1 OBJ: 2 PTS: 1 34. ANS: five DIF: 1 REF: 2 OBJ: 1 PTS: 1 35. ANS: DIF: 1 REF: 3 OBJ: 3 group family PTS: 1 DIF: basic REF: chapter 18 | section 18.3 MATCHING 36. 37. 38. 39. 40. ANS: ANS: ANS: ANS: ANS: F A C B D PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 DIF: DIF: DIF: DIF: DIF: basic basic basic basic basic REF: REF: REF: REF: REF: chapter 17 | section 17.1 chapter 17 | section 17.1 chapter 17 | section 17.1 chapter 17 | section 17.1 chapter 17 | section 17.1 41. 42. 43. 44. 45. ANS: ANS: ANS: ANS: ANS: D B A E C PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 DIF: DIF: DIF: DIF: DIF: basic basic basic basic basic REF: REF: REF: REF: REF: chapter 18 | section 18.1 chapter 18 | section 18.1 chapter 18 | section 18.1 chapter 18 | section 18.1 chapter 18 | section 18.1 46. 47. 48. 49. ANS: ANS: ANS: ANS: C D B A PTS: PTS: PTS: PTS: 1 1 1 1 DIF: DIF: DIF: DIF: basic basic basic basic REF: REF: REF: REF: chapter 18 | section 18.2 chapter 18 | section 18.2 chapter 18 | section 18.2 chapter 18 | section 18.2 SHORT ANSWER 50. ANS: A. carbon B. 13 C. 136 C PTS: 1 51. ANS: DIF: advanced REF: chapter 18 | section 18.2 The neutral fluorine atom has one filled energy level and seven electrons left over. PTS: 1 DIF: advanced REF: chapter 18 | section 18.3 ESSAY 52. ANS: Student 1's results cluster together closely, so they have relatively high precision. However, they are not clustered about the correct value, 10.0, but are significantly off, so they are relatively inaccurate. Student 2's results do not cluster together closely, so they are imprecise. However, they are averaged around the correct value, 10.0, so as a set of data they are fairly accurate. Student 3's results are far apart and are far off the correct value, even when averaged, so they are both imprecise and inaccurate. Student 4's results cluster together closely and are close to the correct value, so they are both precise and accurate. PTS: 1 53. ANS: OBJ: 1A 1.f (a) five; (b) three (the two zeros are not significant); (c) two (the three zeros are not significant); (d) four; (e) six PTS: 1 54. ANS: OBJ: 1A 1.g volume = length width height = 27.0 cm 14.55 cm 9.3 cm = 3653.505 cm3, which must be rounded to two significant digits to match the uncertainty in 9.3 cm, so the final answer should be reported, after rounding, as 3700 cm3, or 3.7 103 cm3. PTS: 1 55. ANS: OBJ: 1A 1.g PTS: 1 56. ANS: OBJ: 1A 1.h PTS: 1 57. ANS: OBJ: 1A 1.h PTS: 1 58. ANS: OBJ: 1A 1.i PTS: 1 59. ANS: OBJ: 1A 1.j PTS: 1 60. ANS: OBJ: 1A 1.j PTS: 1 61. ANS: OBJ: 1A 1.j Precision and accuracy are both evaluations of the reliability of measured values. Precision refers to repeatability of values, or closeness compared to one another. Accuracy refers to the closeness of values to the correct or standard value. PTS: 1 OBJ: 1A 1.f