Disclaimer - American Society of Exercise Physiologists
advertisement

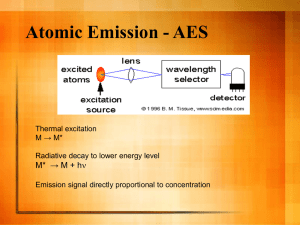
72 Journal of Exercise Physiologyonline August 2014 Volume 17 Number 4 Editor-in-Chief Official Research Journal of Tommy the American Boone, PhD, Society MBAof Review Board Exercise Physiologists Todd Astorino, PhD Julien Baker, ISSN 1097-9751 PhD Steve Brock, PhD Lance Dalleck, PhD Eric Goulet, PhD Robert Gotshall, PhD Alexander Hutchison, PhD M. Knight-Maloney, PhD Len Kravitz, PhD James Laskin, PhD Yit Aun Lim, PhD Lonnie Lowery, PhD Derek Marks, PhD Cristine Mermier, PhD Robert Robergs, PhD Chantal Vella, PhD Dale Wagner, PhD Frank Wyatt, PhD Ben Zhou, PhD Official Research Journal of the American Society of Exercise Physiologists ISSN 1097-9751 JEPonline The Importance of Adjustments for Changes in Plasma Volume in the Interpretation of Hematological and Inflammatory Responses after Resistance Exercise André de Oliveira Teixeira,1 Ozeia Simões Franco,1 Maicon Moraes Borges,1 Cassio Noronha Martins,1 Luis Fernando Guerreiro,1 Carlos Eduardo da Rosa,1 Felipe da Silva Paulitsch,1 William Perez,2 Antonio Marcos Vargas da Silva,3 Luis Ulisses Signori1,3 1Federal University of Rio Grande, Rio Grande, RS, Brazil, 2Federal University of Pelotas, Pelotas, RS, Brazil, 3Federal University of de Santa Maria, Santa Maria, RS, Brazil ABSTRACT Teixeira AO, Franco OS, Borges MM, Martins CN, Guerreiro LF, da Rosa CE, Paulitsch FS, Perez W, Silva AMV, Signori LU. The Importance of Adjustments for Changes in Plasma Volume in the Interpretation of Hematological and Inflammatory Responses after Resistance Exercise. JEPonline 2014;17(4):72-83. This study evaluated hematological and inflammatory alterations resulting from an acute session of resistance exercises (RE) and corrected them by plasmatic volume adjustments. Subjects consisted of 31 men who were not practicing bodybuilding. The RE session consisted of 4 sets of 10 repetitions maximum for each exercise (extensor bench, the squat and leg press) with an interval of 1 min between sets and 2 min between exercises. The concentration of erythrocytes, platelets, total leukocytes (and fractions), ultrasensitive CRP, creatinine kinase (CK), and fibrinogen was evaluated in the blood before (basal) and after the RE. Plasmatic volume alterations were calculated by hematocrit variations. Immediately after the RE, a rise was observed in erythrocytes (6%), platelets (20%), total leukocytes (25%), segmented neutrophils (25%) and rods (18%), monocytes (44%), lymphocytes (17%), CK (15%), and CRP (23%). Percent change in plasma volume was -7.5% (P<0.001). After adjustment by the reduction of plasma volume, the erythrocytes, neutrophils rods, lymphocytes, CK, and CRP revealed values similar to basal levels (P>0.05). Plasmatic volume adjustments interfered in the interpretation of leukocyte and inflammatory response immediately after the RE. Key Words: Exercise, Inflammation, Plasma Volume 73 INTRODUCTION Resistance exercises (RE) induce mechanical, metabolic, and hormonal alterations that chronically interfere in weight control, dyslipidemia, glucose permeability, and immunological resistance. The result is a reduction in risk factors associated with sedentary habits (9). However, when the exercises are acutely performed at high intensity, they trigger an inflammatory response. This outcome is due to alterations in the cellular components, leading to lesions in the contractile and conjunctive tissues (4). The inflammatory response occurs due to excessive production of reactive oxygen and nitrogen species that activate chemical mediators and adhesion molecules in the endothelium (23), favoring attraction, adhesion and migration of leukocytes to the injured tissue (15). The RE, especially when practiced at high intensity, induces homeostasis alterations that lead to a reorganization of several affected tissue responses, including the hematopoietic one (27). The increase of blood reactivity can be observed during and after the exercises (6), and it is characterized by the increase in the concentrations and/or the activity of inflammatory markers of acute phase-proteins (7), platelets, erythrocytes (6), and leukocytes (27). Concomitantly with the cellular and inflammatory responses induced by the RE, variations in the plasmatic volume occur (2,22,24) that may interfere in the results of these variables. There are different methods to measuring these variations when challenged by different types of exercises (18). The most commonly used are the indirect methods based on predictive equations that adopt blood components (hematocrit, hemoglobin, and albumin) to estimate the relative changes in the plasmatic volume (18,28). Studies concerning hematological responses after RE must consider the plasmatic volume reduction (2,22,24), which leads to an increase in blood viscosity (6) and hemoconcentration (2) that in turn partially contributes to leukocytosis and raises in inflammatory markers. However, studies employing correction factors for volume alterations in the hematological and inflammatory variables after RE remain incomplete. The aim of the present research was to verify the alterations caused in blood cells and inflammatory markers after a protocol of RE and correct the results by alterations in the plasmatic volume. METHODS The project was approved by the Research Ethics Committee in the Health Area at the Federal University do Rio Grande (FURG), protocol number 23116.002536/2010. Data were collected in the weight room of our institution. Each subject gave a written informed consent in order to be able to participate in our study, which was approved by the ethics committee of the institution in accordance to the Resolution (n. 196/96) of the National Health Council. Subjects On the day of the examination, the volunteers who had ultrasensitive CRP (C-reactive protein) >3 mg·dL-1, leukocytosis (>11000 x103/mm3), hyperthermia (>38ºC), changes in the systemic blood pressure (>140/90 mmHg), any pain or discomfort symptom, dyslipidemia, and glucose above 100 mg·dL-1 were excluded from the study. Initially, 35 volunteers were selected and provided instructions regarding the experimental protocols and RE. The informed consent form was signed before any testing. All volunteers were previously evaluated by an assistant physician who was invited to participate in the study. The selected subjects consisted of 31 male subjects between 20 and 35 yrs old. They were clinically healthy with a body mass index lower than 30 kg·m-2. Each subject had 74 practiced bodybuilding, but during this study was not practicing any physical and/or regular physical activities. In addition, the subjects were not engaged in any diet programs, and they had no previous diagnosis of rheumatic, cardiovascular, metabolic, neurological, oncological, immunological, and hematologic diseases. Smokers or individuals using any type of medication, diet, vitamin, and/or ergogenic supplements were not included. The subjects were told to maintain their daily routine and eating habits. The consumption of fruit juice and alcoholic beverages, as well as the practice of physical exercises were restricted to 72 hrs before data collection. Procedures Muscular Strength Assessment A test composed of 10 repetitions maximum (10 RM) was adopted in order to perform under training protocols, using a controlled overload (13). The selected exercises were the extensor bench, the squat and the leg press. Previous to the strength test, the subjects were submitted to a specific warm up for each exercise (a series of 15 repetitions with 40% to be used in the first attempt of each test, for each exercise respectively). During data collection, a recovery time of 5 min between the exercises was adopted. Workload values in the 10 RM test were reached in 3 to 5 attempts, when the subject expressed a concentric fault for the dynamic movement. Progressive increments were made using 5 kg in each new attempt with an interval of 3 to 4 min between each exercise series. Therefore, the last exercise was validated as the maximum load. Exercise Session Prior to the exercise session, the subjects were submitted to specific warm ups for each exercise adopted (a series of 15 repetitions with 40% of the load Max obtained in the 10 RM test). The exercise sessions were comprised of 4 series of 10 RM with an interval of 1 min between series and 2 min between exercises. The sequence of exercises (extensor bench, squat and leg press) was randomized by the drawing of sealed brown envelopes. Before the 10 RM test and the data collection, standard instructions were given concerning the experimental procedure and execution technique of the exercises. Verbal stimuli to the subjects were given during the assessment and exercises. Biochemical Measurements Blood collection took place before the exercises and immediately 30 min after completing the RE protocol. The levels of total cholesterol, triglycerides, high density lipoproteins (HDLc), glucose, uric acid, and urea were assessed using LAB TEST commercial tests (Lagoa Santa, MG, Brazil) and analyzed by the LAB MAX 240® device (Tokyo, Japan). The low density lipoproteins (LDLc) were calculated using Friedewald’s formula. The fibrinogen was analyzed by the START device model (Diagnóstica Stago, Asnieres, France) with LAB TEST commercial kits (Lagoa Santa, MG, Brazil). The serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) were measured by the IFCC method (Hitachi 917® device, Roche Diagnostics, Florida, USA). Creatine kinase (CK) measurement was performed using CK-NAC Liquiform reactive (Mindray, model BS200, China). The ultrasensitive C-reactive protein (CRP) was assessed by Nephelometry (Nephelometer Beckman Coulter, Immage model with laboratory reagents CCRP Immage, Fullerton, CA, USA). Lactate was evaluated through tapes (Roche Diagnostics GmbH, Mannheim, Germany) and was analyzed by the Accutrend PLUS device (Roche, Schweiz, Switzerland). Red blood cell and differential white blood cell counts were carried out automatically (ABX kits, Horiba Diagnostics, Curitiba, PR, Brazil) and by microscopy. Regarding the measurement of hematological variables, the samples were counted twice and values expressed by the average of the measurements. When a difference higher than 10% between the two results was observed, the procedure was repeated. 75 Changes in Plasma Volume Percentage changes in plasma volume (%∆PV) were calculated using Van Beaumont’s equation (1972) - %∆PV = [100/(100-Hct Pre)]x[(100(Hct Pre – Hct Post))/Hct Post] - where %∆PV = percent change in plasma volume, Hct Pre = hematocrit value Pre RE, and Hct Post = hematocrit value immediately after RE. This equation was applied in previous studies to estimate variations in plasmatic volume after RE (22). The values of hematological and inflammatory markers were adjusted for plasma volume changes using the following formula: Corrected value = Uncorrected value x ((100 + %∆PV)/100) (24). The %∆VP was applied as a correction factor for each variable (hematological and inflammatory) individually and the results are shown as values after RE corrected by the percentage change in plasma volume (RE-%PV). Statistical Analyses The data are expressed as means and standard deviations (SD). Variations between groups are presented as mean differences and 95% confidence intervals (CI) for those differences. The Kolmogorov-Smirnov test was used to check data distribution. Student’s paired t-test was used to compare the variables with two measurements. Variables with more than two measurements were analyzed by one-way ANOVA for repeated measurements or by Friedman’s ANOVA followed by Bonferroni post hoc test. Statistical significance was set at P≤0.05. RESULTS Table 1 shows the physical and metabolic characteristics from the 31 subjects in the study. The mean age of the subjects was 26.2 ± 4 yrs old with a body mass index lower than the obesity criteria (25.4 ± 3 kg·m-2). Their resting blood pressure was in the normal range for their age. Plasmatic glucose levels, total cholesterol, triglycerides, LDLc, HDLc, urea, uric acid, GOT, and GPT were fitted into normal parameters. Table 1. Clinical and Fasting Metabolic Characteristics of the Subjects (M ± SD). Characteristic Mean ± SD Age (yrs) Body Mass Index (kg·m-2) Systolic Blood Pressure (mmHg) Diastolic Blood Pressure (mmHg) Plasma Glucose (mg·dl-1) Total Cholesterol (mg·dl-1) Triglycerides (mg·dl-1) High-density Lipoprotein Cholesterol (mg·dl-1) Low-density Lipoprotein Cholesterol (mg·dl-1) Urea (mg·dl-1) Uric Acid (mg·dl-1) Glutamic-Oxaloacetic Transaminase (U/L) Glutamic-Pyruvic Transaminase (U/L) 26.3 4 25.4 3 118.2 5 80.3 4 85.1 10 145.0 32 100.9 65 34.6 7 89.9 26 29.9 8 4.8 1 29.0 9 32.1 10 The basal hematocrit values (47.6 ± 2.1 mL/%) were within normal parameters and these increased approximately 5% (P<0.001) after the RE (RE: 49.6 ± 2.9 mL/%). However, 30 min after the RE (47.5 ± 2.4 mL/%), these values were reduced compared to the evaluation carried out immediately after the 76 RE (P<0.001) and returned to basal levels (P=0.765). Application of Van Beaumont’s equation (1972) (28) revealed a reduction of 7.5% ± 6.5 in plasma volume, but this effect was no longer observed 30 min after the end of RE (Figure 1). Figure 1. Resistance Exercise (0 and 30 min) Effects on Plasma Volume (%). The data are expressed as mean ± SD. min: minutes RE: Resistance exercise. RE-%PV: values after resistance exercises corrected by the percent change in plasma volume (%∆PV). *P<0.05 vs. basal. The values of plasmatic levels from the blood cells during the experiment are presented in Figure 2. The erythrocytes (Figure 2A) increased 6% (P<0.001, mean difference 0.247 x105/mm3, 95% CI: 0.139 to 0.356) and returned to basal levels after plasma volume correction. Platelets (Figure 2B) increased 20% (P<0.001, mean difference 46 x103/mm3, 95% CI: 31 to 60) and did not return to basal levels after correction. Total leukocytes (P<0.001, mean difference 1610 x103/mm3, 95% CI: 1196 to 2024) and segmented neutrophils (P<0.001, mean difference 970 x103/mm3, 95% CI: 735 to 1205) were increased approximately 25% and rod neutrophils 18% (P<0.01, mean difference 14 x103/mm3, 95% CI: 4 to 24). After correction by the plasma volume decrease, the total leukocytes (P<0.001, mean difference 701 x103/mm3, 95% CI: 289 to 1115; Figure 2C) and the segmented neutrophils (P<0.001, mean difference 394 x103/mm3, 95% CI: 160 to 629; Figure 2D) remained with an increase of 15% and 18% (P<0.001), respectively, whereas young neutrophils (rods) returned to basal values (Figure 2E). The monocytes were increased after the RE (P<0.001, mean difference 136 x103/mm3, 95% CI: 91 to 180) and, after applying the correction factor, this variable remained 48% above basal levels (P<0.001, mean difference 99 x103/mm3, 95% CI: 55 to 146; Figure 2F). The basophils (less than 1%) and eosinophils were not modified along the experiment (Figure 2G). The resistance exercises induced an apparent lymphocytosis (P<0.001, mean difference 519 x103/mm3, 95% CI: 325 to 713; Figure 2H) of 17%, although after plasma volume correction these values returned to basal levels. 77 Figure 2. Erythrocytes, Platelets and Total Leukocytes Values and its Fractions Corrected by the Percentage of Plasma Volume. The data are expressed as mean ± SD. RE: Resistance exercise. RE-%PV: values after resistance exercises corrected by the percent change in plasma volume (%∆PV). *P<0.05 vs. basal; †P<0.05 vs. RE. 78 The inflammatory markers evaluated during the experiment are shown in Figure 3. Fibrinogen plasma concentrations were not modified (Figure 3A). The RE caused an increase of 15% in CK (P<0.005, mean difference 25 U/L, 95% CI: 8 to 42; Figure 3B), and after correction of plasma volume alterations, the values were no longer significant when compared with basal values. After the RE, CRP increased 23% (P<0.01, mean difference 0.21 mg·dL-1, 95% CI: 0.06 to 0.35; Figure 3C) and plasma volume correction returned this variable to basal values. The RE caused a 5-fold increase in lactate (P<0.001, mean difference 8.5 mmol·dL-1, 95% CI: 8.1 to 9.0), after correction by changes in plasma volume the values reduced in comparison to RE, however, this modification did not cause a return to basal values (P<0.001, mean difference 7.8 mmol·dL-1, 95% CI: 7.3 to 8.3; Figure 3D). Figure 3. Change in Inflammatory Markers Corrected by the Percentage of Plasma Volume. The data are expressed as mean ± SD. RE: Resistance exercise. RE-%PV: values after resistance exercises corrected by the percent change in plasma volume (%∆PV). *P<0.05 vs. basal; †P<0.05 vs. RE. DISCUSSION The resistance exercises induce an expected rise of inflammatory markers (CRP and CK), total leukocytes and their fractions. On the other hand, the collections that were executed immediately after the end of the RE displayed a reduced plasmatic volume, favoring the hemoconcentration. The significant variation previously observed in the young neutrophils and in the lymphocytes perceptual, as well as the CK and CRP were not detected after plasma volume correction. 79 The hematological and inflammatory alterations are a result of many mechanisms. Among these, there is the muscle contraction and relaxation that causes the increase in ischemia and repercussion (4). The end result is an altered oxygen supply with consequent reactive species of oxygen and nitrogen formation (23). Added to these factors is the metabolic stress resulting from RE (10). The tissue antioxidant capacity (enzymatic and non-enzymatic) protects against reactive species of oxygen and nitrogen formation, thus keeping the cell redox balance and avoiding a state of oxidative stress. This state is characterized by a higher reactive species of oxygen and nitrogen formation, due to a deficient antioxidant capacity, and may encourage lesion in cellular components and/or attenuate as signals or as a part of the triggering mechanism of inflammatory response (3,12). The present study demonstrates an acute reduction of 7.3% in plasma volume immediately after the exercises. The results are in accordance with previous studies that have shown a decrease of plasma volume between 2.5% (22) and 10% (2,24). Additionally, these alterations are dependent on the training variables involved in the RE session (25) as well as the type of exercise (5). Ahmadizad and El-Sayed (2) have shown that the mentioned plasma volume reduction caused an increase in erythrocytes (5.6%), hemoglobin (5.4%), and hematocrit (6.2%), as observed in the present study. Nevertheless, after plasma volume correction, the erythrocytes returned to basal levels. Such a fact occurs because the erythrocytes are the most abundant cells and are inversely proportional to the variations of hematocrit, which was the variable used to estimate the plasmatic variations (28). The exercises increased the rigidity and agreeability of the erythrocytes (6). The concentration of blood platelets increased in 20% and did not return to basal values after plasma volume correction. This finding suggests that an acute session of RE increases blood coagulation, which is in agreement with a previous study demonstrating that the RE promoted the activation and reduction of the platelets aggregation time (1). Our results suggest that leukocytosis after the RE occurs due to an increase in the neutrophilia and monocytosis, once the plasma volume correction has shown that the rod neutrophils (young) and the lymphocytes were not increased when compared with the basal collection. These results are in accordance with previous studies, once the increase of circulating leukocytes is observed during and immediately after the RE accomplishment (27). Leukocytosis occurs mainly due to neutrophilia (29) and monocytosis (20) of which both are caused by the release and recruitment of these cells at different tissues. Lymphocytosis and the increase of young neutrophils were already tested in previous studies (26,29). Nevertheless, these alterations were more evident after plasma volume correction in the present study. We believe that the time of duration of the RE protocol applied in our study is enough to stimulate the bone marrow and the lymphatic system in order to produce and/or release new cells, that is, not dismissing the possibility of protocols with higher duration to present different responses of these cells. Additionally, the RE increased the plasma concentration of CK, which returned to normal levels after plasma volume correction. The presence of this enzyme in plasma is a muscle damage marker that increases immediately after the RE (17). However, higher levels are normally found between 24 and 72 hrs after the RE (21). The CRP increased immediately after the protocol of the exercises and returned to basal values after plasma volume correction. The increase immediately after the RE was already demonstrated (17), although these alterations occur few hours after the RE (14). This systemic nonspecific inflammatory marker is produced by the hepatocytes, stimulated by IL-6 (11), that is produced in the adipose tissue and mononuclear blood cells as a response to TNF-α and IL-1β from the inflamed tissues (19). The CRP increases observed in blood are normally observed 24 hrs after long duration and/or high intensity exercises (19) and the increases occur independently of the exercise modality (16). The oxidation of circulating neutrophils would be the most acceptable 80 mechanism by which CRP is increased immediately after the RE (17). Therefore, we believe that the CK and CRP measures, immediately after the RE without plasma volume correction, were shown to be limited as inflammatory markers. The fibrinogen plasmatic concentrations were not altered after the RE. This finding is in agreement with Clark et al. (8) who observed that the RE of high (30% of 1 RM) and low (80% of 1 RM) intensities targeting lower limbs did not modify this inflammatory marker. The lactate remained increased after hemoconcentration correction. Zouhal et al. (31) studied recovery (5, 10, and 20 min) after running (endurance and sprint) and observed that plasma correction did not change the observed increase of lactate, which is in agreement with our results. We emphasize that this variable is an indicator of the exercise intensity and, concerning our study, the plasma volume correction presented an insignificant interference in the response. Limitation of the Study The results of the present study should not be extrapolated for other exercise protocols, once these homeostatic alterations are modulated depending on the training variables (such as load, volume, recovering interval, number of repetitions, frequency, velocity, and execution order of the RE), which were not investigated (25). The method used for hematocrit evaluation (manual or automated) shows differences of 2.5% in the results, which may have an impact in the alterations of the calculated plasma volume (30). Thus, this might be one of the limitations of the study. CONCLUSIONS The plasma volume correction must be considered in studies concerning hematological evaluations carried out immediately after sessions of RE, especially since failing to do so may lead to improper interpretations of these variables results. These alterations disappear before half an hour after the end of the exercises, suggesting that the data collected after this period presents more reliable interpretations. The variables that are previously corrected by the protein and/or lipid sample contents are also more reliable in this condition. ACKNOWLEDGMENTS The present study had support from the National Council of Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) and the Research Support Foundation from Rio Grande do Sul State (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul - FAPERGS). We would like to thank the staff at the Clinical Analysis Laboratory from the Charity Association of the Santa Casa Hospital in Rio Grande, the Rouget Perez Laboratory in Pelotas and the Biological Sciences Institute – FURG which collaborated in data analysis and processing. Address for correspondence: Luis Ulisses Signori, PhD, Instituto de Ciências Biológicas, Universidade Federal do Rio Grande - FURG, Av. Itália km 8 - Campus Carreiros; ZIP CODE: 96.201-900 - Rio Grande - RS – Brazil, E-mail: l.signori@hotmail.com 81 REFERENCES 1. Ahmadizad S, El-Sayed MS, MacLaren DP. Effects of time of day and acute resistance exercise on platelet activation and function. Lin Hemorheol Microcirc. 2010;45(2-4):391-399. 2. Ahmadizad S, El-Sayed MS. The acute effects of resistance exercise on the main determinants of blood rheology. J Sports Sci. 2005;23(3):243-249. 3. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9-11. 4. Bloomer RH, Goldfarb AH. Anaerobic exercise and oxidative stress: A review. Can J Appl Physiol. 2004;29(3):245-402. 5. Bloomer RJ, Farney TM. Acute plasma volume change with high intensity sprint exercise. J Strength Cond Res. 2013;27(10):2874-2878. 6. Brun JF, Khaled S, Raynaud E, Bouix D, Micallef JP, Orsetti A. The triphasic effects of exercise on blood rheology: With relevance to physiology and pathophysiology? Clin Hemorheol Microcirc. 1998;19(2):89-104. 7. Ceciliane F, Giordano A, Spagnolo V. The systemic reaction during inflammation: The acutephase proteins. Protein Pept Lett. 2002,9:211-223. 8. Clark BC, Manini TM, Hoffman RL, Williams PS, Guiler MK, Knutson MJ, et al. Relative safety of 4 weeks of blood flow-restricted resistance exercise in young, healthy adults. Scand J Med Sci Sports. 2011;21:653–662. 9. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American College of Sports Medicine American. College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459-471. 10. Hudson MB, Hosick PA, McCaulley GO, Schrieber L, Wrieden J, McAnulty SR, et al. The effect of resistance exercise on humoral markers of oxidative stress. Med Sci Sports Exerc. 2008; 40(3):542-548. 11. Johnson TV, Master VA, Michigan A. Review of the relationship between C-reative protein and exercise. Mol Diagn Ther. 2011;15(5):265-275. 12. Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chem Biol Interact. 2006;163:38-53. 13. Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, et al. American College of Sports Medicine Position Stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34(2):364-380. 14. Krüger K, Agnischock S, Lechtermann A, Tiwari S, Mishra M, Pilat C, et al. Intensive resistance exercise induces lymphocyte apoptosis via cortisol and glucocorticoid receptordependent pathways. J Appl Physiol. 2011;110(5):1226-1232. 82 15. McCarthy DA, Dale MM. The leucocytosis of exercise. A review and model. Sports Med. 1988;6:333-363. 16. Mendham AE, Donges CE, Liberts EA, Duffield R. Effects of mode and intensity on the acute exercise-induced IL-6 and CRP responses in a sedentary, overweight population. Eur J App Physiol. 2011;111(6):1035-1045. 17. Nakajima T, Kurano M, Hasegawa T, Takano H, Lida H, Yasuda T, et al. Pentraxin3 and highsensive C-reative protein are independent inflammatory markers released during high-intensity exercise. Eur J App Physiol. 2010;110(5):905-913. 18. Novosadová J. The changes in hematocrit, hemoglobin, plasma volume and proteins during and after different types of exercise. Eur J App Physiol. 1977;37:223-230. 19. Petersen AMW, Pedersen BK. The anti-inflammatory effect if exercise. J Appl Physiol. 2005; 98:1154-1162. 20. Pournot H, Bieuzen F, Louis J, Fillard J-R, Baarbiche E, Hausswirth C. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS One. 2011;6:e22748. 21. Quindry J, Miller L, McGinnis G, Irwin M, Dumke C, Magal M, et al. Muscle-fiber type and blood oxidative stress after eccentric exercise. Int J Sport Nutr Exerc Metab. 2011;21(6):462-470. 22. Ramel A, Wagner K, Elmadfa I. Plasma antioxidants and lipid oxidation after submaximal resistance exercise in men. Eur J Nutr. 2004;43:2-6. 23. Rietjens SJ, Beelen M, Koopman R, Van Loon LJ, Bast A, Haenen GR. A single session of resistance exercise induces oxidative damage in untrained men. Med Sci Sports Exerc. 2007; 39(12):2145-2151. 24. Sherk DV, Chrisman C, Smith J, Young KC., Singh H, Bemben MG, Bemben DA. Acute bone marker responses to whole-body vibration and resistance exercise in young women. J Clin Densitom. 2013;16(1):104-109. 25. Simão R, Salles BF, Figueiredo T, Dias I, Willardson JM. Exercise order in resistance training. Sports Med. 2012;42(3):251-265. 26. Simonson SR, Jackson CG. Leukocytosis occurs in response to resistance exercise in men. J Strength Cond Res. 2004;18(2):266-271. 27. Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6-48. 28. Van Beaumont W. Evaluation of hemoconcentration from hematocrit measurements. J Appl Physiol. 1972;32:712-723. 29. Wang JS, Huang YH. Effects of exercise intensity on lymphocyte a apoptosis induced by oxidative stress in men. Eur J App Physiol. 2005;95:290-297. 83 30. Watson P, Maughan RJ. Artifacts in plasma volume changes due to hematology analyzerderived hematocrit. Med Sci Sports Exerc. 2014;46(1):52-59. 31. Zouhal H, Vincent S, Moussa E, Jacob C, Groussard C, Ben Abderrahaman A, Prioux, et al. Influence of training status on plasma volume variations and plasma lactate concentrations in response to supramaximal exercise. Biology of Sport. 2007;24(4). Disclaimer The opinions expressed in JEPonline are those of the authors and are not attributable to JEPonline, the editorial staff or the ASEP organization.