Six membered heteroc..
advertisement
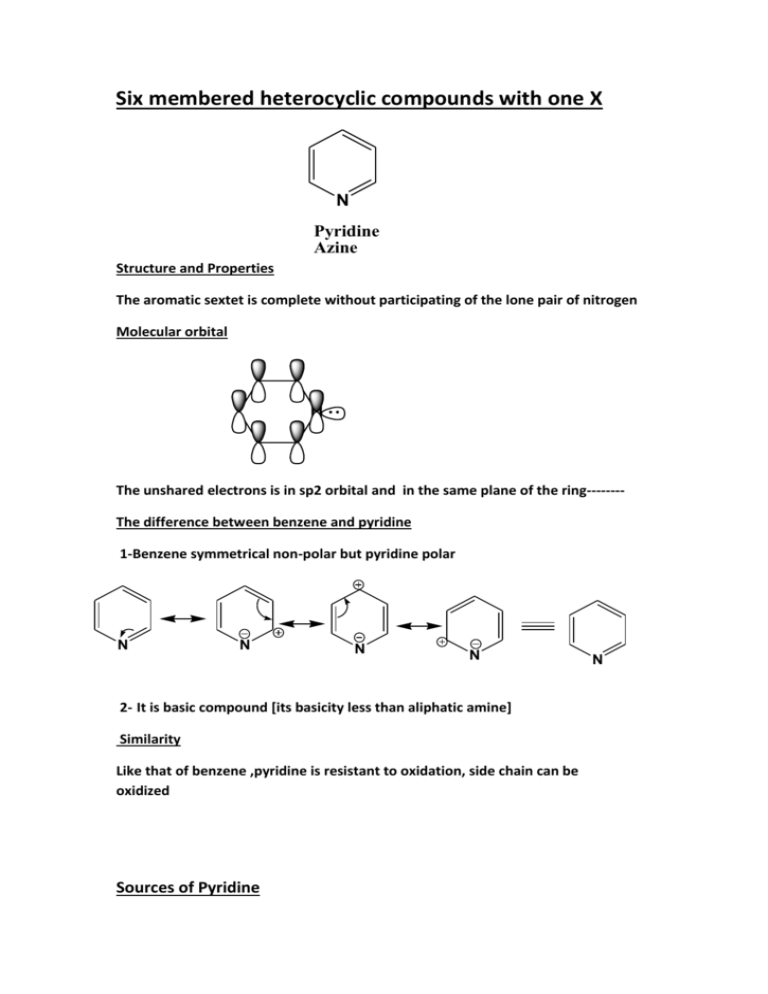
Six membered heterocyclic compounds with one X N Pyridine Azine Structure and Properties The aromatic sextet is complete without participating of the lone pair of nitrogen Molecular orbital N The unshared electrons is in sp2 orbital and in the same plane of the ring-------The difference between benzene and pyridine 1-Benzene symmetrical non-polar but pyridine polar N N N N 2- It is basic compound [its basicity less than aliphatic amine] Similarity Like that of benzene ,pyridine is resistant to oxidation, side chain can be oxidized Sources of Pyridine N Found in coal tar CH3 CH3 N N CH3 CH3 N CH3 N picoline picoline picoline CH3 H3C N 2,4-lutidine CH3 2,4,6-collidine Oxidation of picolines yields the pyridine carboxylic acids CH3 COOH KMnO4 N N Synthesis of Pyridine Hantzsch pyridine synthesis H EtO O H EtO O + + O O NH3 or NH4OAc O O H H Et N H O O OEt CH3 O CH3 Reaction of Pyridine A: Electrophilic Substitution SE Et OEt FeCl3 H2O H3C N CH3 Accure chiefly at the 3-position NO2 HNO3, H2SO4 300 C N 3- nitropyridine SO3H H2SO4 350 C N 3-pyridine sulfonic acid Br N Br Br Br2 300 C N 3- bromopyridine RX or RCOX AlCl3 No Reaction + N 3,5-dibromopyridine H E H E H E C-4 attack N N N not favoured C-2 attack H N N H E H E N E N not favoured H C-3 attack H E N H E N E N Give Resone In contast to benzene, Pyridine is very unreactive to SE reaction ? B: Neucleophilic Substitution SN Accure at C-2 and C-4 position H Nu H Nu H Nu C-4 attack N N N specially stable C-2 attack H N N H Nu H Nu N Nu N specially stable H C-3 attack H Nu H Nu Nu N N N Examples 1- Chichibabin Reaction NaNH2 N NH2 N Na+ H stabilized intrmediate - NaH N 2-aminopyridine NH2 2- Alkylation or Arylation Reaction by Organolithium compounds Ph-Li+ N Li+ H N NaOH - Cl- Cl Cl N Ph 2-phenylpyridine stabilized intermediate N + LiH heat heat N - NaH Ph OH N OH N H O 2-hydroxylpyridine stabilized intermediate 2-pyridone enol keto Br NH2 Br Br Br Br - Br- NH3 N NH2 N stabilized intermediate N C: Reaction at N-1 1- alkylation CH3 H3C CH3I Br CH3 N H3C + N N I C CH2 H3C H CH3 N-methyl pyridinium iodide 2- Acylation O O-Cl Ph-C N Ph S O Cl N N O C Cl Ph benzoyl chloride O S O Cl Ph 3- Rx with peracids and with lewis acid AlC -H OO -C O CH 3 H+ COO H 2O 2 N l3 CH 3 N N AlCl3 O Pyridine -N-Oxide To activate SE N O To activate SN N O Examples Electrophilic ( Nitration) H NO2 HNO3\H2SO4 NO2 NO2 PCl3 H+ + POCl3 heat N N N O O O N Stabilized intermediate Neucleophilic NO2 H3CO NaOCH3 NO2 OCH3 OCH3 PCl3 -NO2- + POCl3 heat N N N O O O Stabilized intermediate D: Oxidation and Reduction N Reduction H H 2, N pt ,H l C C 25 N a tO \E N H N H 1,4-dihydropyridine Oxidation ?????????????????????????????????? Pipiridine Synthesis of Quinoline Skraup Synthesis CH2OH + CHOH NH2 NO2 + CH2OH H2SO4 FeSO4 + C6H5NH2 + H 2O N Describe the mechanism Bischler Nepieralski Synthesis of isoquinoline CH3COCl NH2 NH O P2O5 heat C CH3 H2 + N N Pd \ heat CH3 CH3 Electrophilic Substitution NO2 O3 c HN \0 O4 S H2 N major Nucleophilic Substitution H KO r o OH Na -L Bu i N OK N Bu NH 2 major H3 Bu 1)K 2 )N N O N H 2-quinolone N minor NH2 N minor Oxidation & Reduction N major NH2 Reduction H2\pt H2\pt N N H LiAlH4 N H cis,trans- decahydro quinoline 1,2,3,4-tetrahydro quinoline 1,2-dihydro quinoline N H Oxidation COOH aq K MnO 4 alk ali ne K N N COOH quinolinic acid M nO 4 COOH neutral KMnO4 COOH O NH O Other fused ring Phthalimide phthalic acid N Acridine Fused Six membered heterocyclic compounds with one X Diazines N N N N N pyrimidine pyridazine pyrazine O NH2 H3C N N H Cytosine C Purines N O O NH NH N H Thyamine T O N H Uracil U O O O O N HN H N N HN HN O N H N O Hypoxanthine N H N H O uric acid Xanthine Cl O H N HN Cl POCl3 Cl N H N H NH2 N N O O N H N H N N Cl N H N aq. NH3 Cl N H N uric acid HI \PH4I 1) HI 2) Zn dust H , aO NH 3 N 1) aq. 2) at he NH2 I O N HN I H2N N N N N N H N N H N N adinine Zn dust guanine N N N H N purine Miscellaneous S N N H N Hypnotic, Sedative, Anxiolytic Anticonvulsant, Muscle relaxant Properties N 1,4-benzodiazepine CH3 O N Valium Preparation of Valium Cl N O NH2 + Cl H N O Cl C C O CH2 C O Cl NH3 H CH3 N N Valium O N O Cl Cl Amino Acids C CH2Cl CH3I N Cl Characteristics and Structures Amino acids contain both a carboxyl group (COOH) and an amino group (NH2). The general formula for an amino acid is given below. Although the neutrally-charged structure is commonly written, it is inaccurate because the acidic COOH and basic NH2 groups react with one another to form an internal salt called a zwitterion. The zwitterion has no net charge; there is one positive (COO-) and one negative (NH3+) charge. There are 20 amino acids derived from proteins. While there are several methods of categorizing them, one of the most common is to group them according to the nature of their side chains. 1-Nonpolar Side Chains 2-Polar, Uncharged Side Chains 3-Charged Side Chains Name Abbreviation Linear Structure Alanine ala A CH3-CH(NH2)-COOH Arginine arg R HN=C(NH2)-NH-(CH2)3-CH(NH2)-COOH Asparagine asn N H2N-CO-CH2-CH(NH2)-COOH Aspartic Acid asp D HOOC-CH2-CH(NH2)-COOH Cysteine cys C HS-CH2-CH(NH2)-COOH Glutamic Acid glu E HOOC-(CH2)2-CH(NH2)-COOH Glutamine gln Q H2N-CO-(CH2)2-CH(NH2)-COOH Glycine gly G NH2-CH2-COOH Histidine his H NH-CH=N-CH=C-CH2-CH(NH2)-COOH Isoleucine ile I CH3-CH2-CH(CH3)-CH(NH2)-COOH Leucine leu L (CH3)2-CH-CH2-CH(NH2)-COOH Lysine lys K H2N-(CH2)4-CH(NH2)-COOH Methionine met M CH3-S-(CH2)2-CH(NH2)-COOH Phenylalanine phe F Ph-CH2-CH(NH2)-COOH Proline pro P NH-(CH2)3-CH-COOH Serine ser S HO-CH2-CH(NH2)-COOH Threonine thr T CH3-CH(OH)-CH(NH2)-COOH Tryptophan trp W Ph-NH-CH=C-CH2-CH(NH2)-COOH Tyrosine tyr Y HO-Ph-CH2-CH(NH2)-COOH Valine val V (CH3)2-CH-CH(NH2)-COOH The 20 Amino Acids The twenty amino acids commonly found in proteins include: nonpolar amino acids: glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile), methionine (met), phenylalanine (phe), tryptophan (trp), and proline (pro) polar amino acids: serine (ser), threonine (thr), cystein (cys), tyrosine (tyr), asparagine (asn), and glytamine (gln) charged amino acids: aspartic acid (asp), glutamic acid (glu), lysine (lys), arginine (arg), and histidine (his) Importance of Amino Acids - Amino acids are the building blocks of protein -you get protein from your diet - 'condensation reactions - polypeptide chains - the individual amino acids are called petides - complex structures know as proteins Essential and Nonessential Amino Acids Nonessential amino acids are those that are synthesized by mammals, while the essential amino acids must be obtained from dietary sources The Ten "Nonessential" Amino Acids Alanine Asparagine Aspartate Cysteine (requires sulfhydryl group from methionine) Glutamate Glutamine Glycine Proline Serine Tyrosine (synthesized from phenylalanine) The Ten "Essential" Amino Acids Arginine (see below) Histidine Isoleucine Leucine Lysine Methionine Phenylalanine Threonine Tryptophan Valine Strecker amino acid synthesis










