OXIDATION NUMBERS (Answer Key)
advertisement

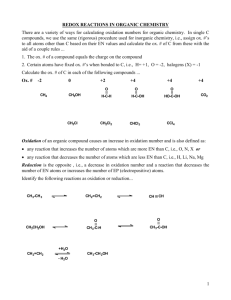
OXIDATION NUMBERS (Answer Key) 1. a. Sn is tin in an uncombined state. The oxidation number is 0. b. The ionic charge on potassium is 1+, thus the oxidation number is +1. c. The ionic charge on sulfur is 2-, thus the oxidation number is -2. d. The ionic charge on iron is 3+, thus the oxidation number is +3. e. Se is selenium in an uncombined state. The oxidation number is 0 f. The ionic charge on magnesium is 2+, thus the oxidation number is +2. g. The ionic charge on tin is 4+, thus the oxidation number is +4. h. The ionic charge on bromine is 1-, thus the oxidation number is -1. 2. a. +3 b. +6 c. +2 d. +6 3. a. Since neither Mg nor Br explicitly show up in the rules sent out on the list, we have to recognize that Mg is an alkali earth metal, and Br is a halogen. We can then use the fact that all alkali earth will be +2 oxidation number, and halogens will be -1 when combined with metals. Checking to see if everything is balanced this way produces: +2 (from Mg) + 2 x -1 (from 2 Br's) = 0. b. From the rules sent out, we know O must be -2, so then we need only determine the oxidation number for Fe. To do so, let's look at what we have from the O: 3 x -2 = -6. We need +6 to balance out, and have 2 Fe atoms to spread the +6 across. Each Fe must therefor be +3. If we check we get: 2 x +3 (from Fe) + 3 x -2 (from O) = 0. c. Since neither Al nor N explicitly show up in the rules we have, we are back to recognizing what is present: a metal+non-metal compound. Oxidation numbers must be assigned by group #, meaning Al will be +3 and N -3. Checking, we get 1 x +3 (from Al) + 1 x -3 (from N) = 0. d. From the rules, we know O must be -2, so we need only determine the oxidation number for S. From the O we have: 3 x -2 = -6. We need +6 to balance out and have one S available, so it will have an oxidation number of +6. Checking, we get 1 x +6 (from S) + 3 x -2 (from O) = 0. e. From the rules, we know O must be -2, so we need only determine the oxidation number for P. However, we must keep in mind the overall charge on the polyatomic ion is -3. From O, we have 4 x -2 = -8. We need -3 overall, so that leaves +5 for the P. Checking, we have 1 x +5 (from P) + 4 x -2 (from O) = -3. f. From the rules, we know O must be -2, so we need only determine the oxidation number for Cr. However, we must keep in mind the overall charge on the polyatomic ion is -2. From O, we have 7 x -2 = -14. We need -2 overall, so that leaves +12 for the two Cr. Each Cr must therefor by +6. Checking, we have 2 x +6 (from Cr) + 7 x -2 (from O) = -2. g. From the rules, we know O must be -2 and H must be +1, so we need only determine the oxidation number for Cl. Normally Cl would be -1 in a compound, but here we will see that is not possible. From the O we have 2 x -2 = -4. From the H we have 1 x +1 = +1. So far, our overall is -3 (-4 + 1), so the Cl cannot be negative since the sum must be 0. Instead, Cl must be +3. Checking, we have 1 x +1 (from H) + 1 x +3 (from Cl) + 2 x -2 (from O) = 0. h. From the rules, we know O must be -2, but that is only part of the battle since it still leaves Cu and S as undefined. The trick here is to recognize SO4 as the sulfate ion which has a 2- charge on it. This tells us the charge on the Cu must be 2+, and therefore that the oxidation number on the Cu is +2 (since oxidation # = charge for elemental ions). Now we only have to determine S, using what we know about SO42-. With 4 x -2 = -8 from O, the S must be +6 in order to get the 2- charge. Checking everything: 1 x +2 (from Cu) + 1 x +6 (from S) + 4 x -2 (from O) = 0.