Electronic Supplementary Material
advertisement

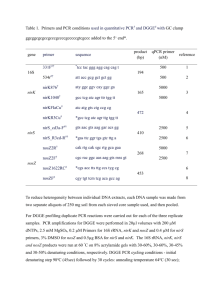
Electronic Supplementary Material Effect of a new synbiotic supplement on symptoms, stool consistency, intestinal transit time and gut microbiota in patients with severe functional constipation: a pilot randomized doubleblind, controlled trial Gabriele Bazzocchi • Tiziana Giovannini • Cristiana Giussani • Patrizia Brigidi • Silvia Turroni G. Bazzocchi • T. Giovannini Montecatone Rehabilitation Institute S.p.A., Imola, Italy C. Giussani Clinical Research Methodologist, Milan, Italy P. Brigidi • S. Turroni Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy Correspondence to: G. Bazzocchi, Montecatone Rehabilitation Institute S.p.A., Via Montecatone, 37 - 40026 Imola, Italy Phone: +39 0542 632800; Fax: +39 0542 632845. E-mail: gabriele.bazzocchi@unibo.it 1 Microbial DNA extraction, PCR-DGGE analysis and quantitative PCR (qPCR) Total microbial DNA was extracted from fecal samples using the QIAamp DNA Stool Mini Kit (QIAGEN, Dusseldorf, Germany) with a modified protocol [1]. In order to create standard lane markers for DGGE gels, the probiotic bacteria claimed in Psyllogel Megafermenti® were isolated by plating serial dilutions on MRS (Merck, Darmstadt, Germany) and HHD [2] agar and incubating in anaerobic jars with AnaerocultA (Merck) at 37°C for 48 h. Chromosomal DNA from probiotics was extracted using the DNeasy Blood & Tissue Kit (QIAGEN), according to the supplier’s instructions. The taxonomic identity of bacterial strains was verified by 16S rDNA gene sequencing using f27 and r1492 universal primers [3]. DNA concentration and quality was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop® Technologies, Wilmington, DE, USA). Due to insufficient DNA material, one patient of Group A was excluded from further analysis. For PCR-DGGE analysis, amplifications of the 16S rDNA gene were carried out using a Biometra Thermal Cycler T Gradient (Biometra, Göttingen, Germany). The following primer sets were used: Bif164/Bif662-GC [4] for Bifidobacterium spp. and Lac1/Lac2-GC [5] for Lactobacillus group (comprising the genera Lactobacillus, Leuconostoc, Pediococcus and Weissella). PCR reaction mixtures contained 0.5 µM of each primer, 200 µM of each dNTP, 2.5 U of GoTaq® Flexi DNA Polymerase (Promega, Milan, Italy) and appropriately diluted template DNA (10-50 ng) in a final volume of 25 µL. The thermocycle program consisted of: initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 1 min, annealing at 61°C (for Lactobacillus group-specific primers) or 62°C (for Bifidobacterium genus-specific primers) for 30 s, extension at 72°C for 40 s and a final extension at 72°C for 7 min. Size and amount of PCR products were estimated by 2% agarose gel electrophoresis. 16S rDNA gene amplicons were analyzed by DGGE as previously described [6]. Amplified DNA from probiotics of Psyllogel Megafermenti ® was run alongside the PCR products from stools as a standard lane marker. Gels were silver-stained [7] and scanned using a Molecular Imager Gel Doc 2 XR System (Bio-Rad, Hercules, CA, USA). DGGE profiles were analyzed using the FPQuest software (v. 4.5, Bio-Rad). qPCR was carried out for fecal specimens from patients of Group A and B using a LightCycler system (Roche, Mannheim, Germany). 16S rDNA gene amplifications were performed in a 20-μL reaction volume containing 50 ng of total bacterial DNA, 4 μL of SYBR Green PCR Master Mix (Roche) and a 500-nM concentration of Bif164/Bif662 [4] primers for Bifidobacterium spp. or Lac1 [5]/Lab0677 [8] for Lactobacillus group. The following cycling profile was used: 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s, 62°C (for Bifidobacterium genus-specific primers) or 65°C (for Lactobacillus group-specific primers) for 20 s, 72°C for 30 s, and an additional 5-s incubation step at 85°C (for Lac1/Lab0677 primer set) or 90°C (for Bif164/Bif662 primer set) for fluorescence acquisition. All sample and primer combinations were run in duplicate. Product detection and PCR specificity were checked post-amplification by examining the temperature-dependent melting curves. Standard curves were constructed using 10-fold serial dilutions of genomic DNA from B. breve or L. plantarum strain isolated from Psyllogel Megafermenti®. Bacterial DNA concentration in stool samples was calculated from the average threshold cycle values (Ct) and expressed as ng of DNA of the targeted group/genus per µg of total DNA extracted from stools. Efficiency of qPCR assays was estimated by the formula E = [10(-1/slope)-1]. References 1. Candela M, Rampelli S, Turroni S et al (2012) Unbalance of intestinal microbiota in atopic children. BMC Microbiol 12:95 2. McDonald LC, McFeeters RF, Daeschel MA, Fleming HP (1987) A differential medium for the enumeration of homofermentative and heterofermentative lactic acid bacteria. Appl Environ Microbiol 53:1382-1384 3. Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons, Chichester, UK, pp 115-175 3 4. Kok RG, de Waal A, Schut F, Welling GW, Weenk G, Hellingwerf KJ (1996) Specific detection and analysis of a probiotic Bifidobacterium strain in infant feces. Appl Environ Microbiol 62:3668-3672 5. Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP (2001) Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using groupspecific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67:2578-2585 6. Vitali B, Cruciani F, Baldassarre ME et al (2012) Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiol 12:236 7. Bassam BJ, Caetano-Anollés G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80-83 8. Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD, de Vos WM (2002) Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol 68:114123 4 Table S1 Demographic and clinical characteristics of patients (mean±SE) Group A Group B 17 12 15 F, 2 M 10 F, 2 M Age (years) 38±12 39±14 BMI (kg/m2) 20±4 18±3 2.4±0.9 2.4±1.1 Patients (no.) Gender Constipation duration (years) 5 Table S2 Presence/absence of DGGE bands matching those of the probiotic strains of Psyllogel Megafermenti® Patient DGGE bands identified as random code L. plantarum L. acidophilus L. rhamnosus B. longum /B. breve 193 + + + + 277 + A + + 323 + + D - 386 + + + + 437 D - D - 535 A + + + 540 - D D - 598 + A + + 751 + D - - 761 + A A - 829 + + + - 831 + A + + 905 + A A A 946 - + + + 955 + + - A 978 + + + + Only patients of Group A were considered. +, present at both baseline and after synbiotic intake. -, absent at both baseline and after synbiotic intake. A, appeared after synbiotic intake. D, disappeared after synbiotic intake. 6