Lesson Plan: Week of 30 November
advertisement

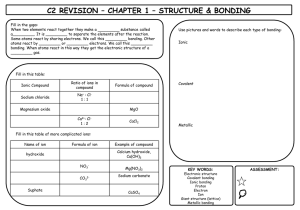
Lesson Plan: Week of 30 November-4 December2015 Subject: MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY ACCRS: Key Ideas and Details: RSL-12-3 and RSL-1-3. Follow precisely a multistep procedure when carrying out experiments, taking measurements/tasks. Key Ideas and Details: RSL-12-3 and RSL-1-3. Follow precisely a multistep procedure when carrying out experiments, taking measurements/tasks. Key Ideas and Details: RSL-12-3 and RSL-1-3. Follow precisely a multistep procedure when carrying out experiments, taking measurements/tasks Key Ideas and Details: RSL-12-3 and RSL-1-3. Follow precisely a multistep procedure when carrying out experiments, taking measurements/tasks Accumulative: Key Ideas and Details. Before: -. -Table Talk: Review Ionic and Covalent Bonding Table Talk: Discuss Tomorrow’s Quiz Topics Substitute Teacher in place Substitute Teacher in place Substitute Teacher in place During: -Lecture on Ionic/Covalent Bonding/Metallic Bonding, Writing Formulas -Lecture on Ionic/Covalent Bonding/Metallic Bonding, Writing Formulas -Lecture on Ionic/Covalent Bonding/Metallic Bonding, Writing Formulas -Lecture on Ionic/Covalent Bonding/Metallic Bonding, Writing Formulas -Lecture on Ionic/Covalent Bonding/Metallic Bonding, Writing Formulas -Lecture on -Lecture on the Mole Concept -Lecture on -Lecture on the Mole Concept -Lecture on -Lecture on the Mole Concept -Lecture on -Lecture on the Mole Concept -Lecture on -Lecture on the Mole Concept RegularChemistry -Lecture on -Lecture on -Lecture on -Lecture on -Lecture on -Lecture on -Lecture on -Lecture on -Lecture on -Lecture on Chemical Reactions and Chemical Reactions and Chemical Reactions and Chemical Reactions and Chemical Reactions and Balancing Equations Balancing Equations Balancing Equations Balancing Equations Balancing Equations After: Exit Slip Exit Slip Desired Outcome: Students will illustrate the differences between ionic and covalent, and metallic bonding. -Students explain why chemical reactions occur. What subatomic particles are most important in determining whether reaction will form and what type of reaction will form? Also included is an explanation how to balance chemical equations, and why is it important . Exit Slip Students will illustrate the differences between ionic and covalent, and metallic bonding. -Students explain why chemical reactions occur. What subatomic particles are most important in determining whether reaction will form and what type of reaction will form? Also included is an explanation how to balance chemical equations, and why is it important . Exit Slip Formative/Summative Assessment - Students will illustrate the differences between ionic and covalent, and metallic bonding. -Students explain why chemical reactions occur. What subatomic particles are most important in determining whether reaction will form and what type of reaction will form? Also included is an explanation how to balance chemical equations, and why is it important . Completed assignment due prior to leaving class. Students will illustrate the differences between ionic and covalent, and metallic bonding. -Students explain why chemical reactions occur. What subatomic particles are most important in determining whether reaction will form and what type of reaction will form? Also included is an explanation how to balance chemical equations, and why is it important . Exit Slip Test Higher Order Questions: There are 4 ways to illustrate ionic bonding, name them? There are 4 ways to illustrate ionic bonding, name them? Name and describe the 5 types of chemical reactions? Explain the steps for balancing chemical reactions/equations. Explain the steps for balancing chemical reactions/equations. Homework: Prep for Friday’s test: Learn the Common Polyatomic Ions on book page 224. Know the formula and name. Read the section on Naming Ions and Ionic compounds, book page 225-226. Complete book questions 88-99, page 272-273 Complete book questions 71-78, pages 304-305. Complete book questions 5-10, page 312. Complete Chapters 711 Comprehensive Review Packet (Due 30 November 15) Complete Chapters 7-11 Comprehensive Review Packet (Due 30 November 15)