Report from Chris Gault, 3rd year Medical Student, Queen`s
advertisement
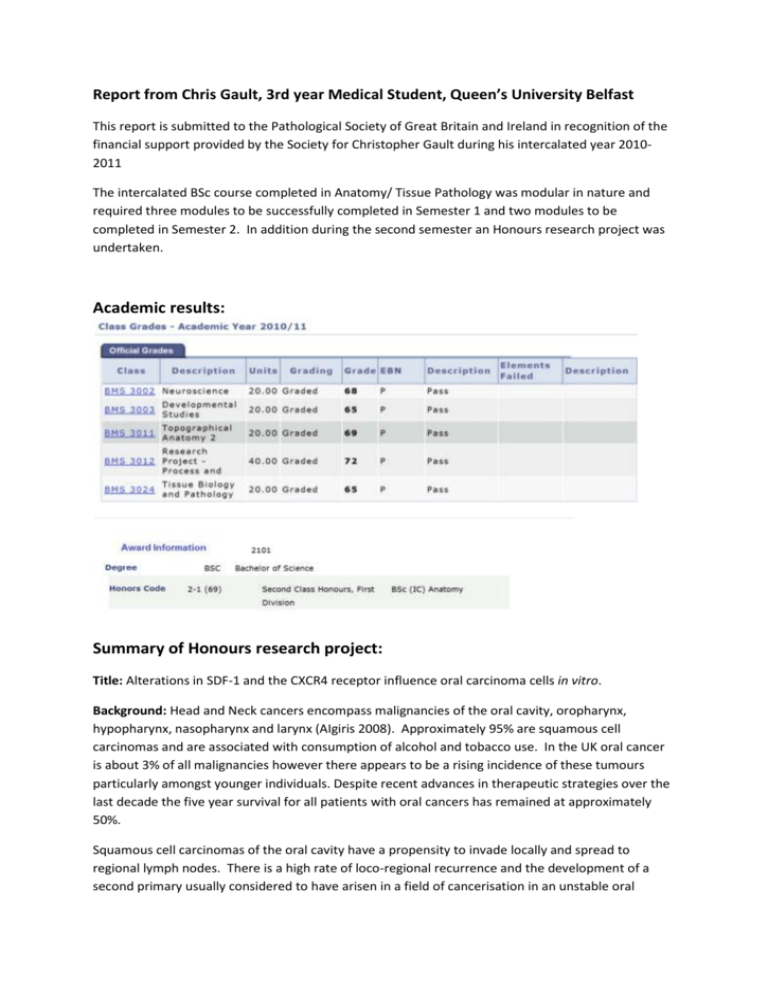
Report from Chris Gault, 3rd year Medical Student, Queen’s University Belfast
This report is submitted to the Pathological Society of Great Britain and Ireland in recognition of the
financial support provided by the Society for Christopher Gault during his intercalated year 20102011
The intercalated BSc course completed in Anatomy/ Tissue Pathology was modular in nature and
required three modules to be successfully completed in Semester 1 and two modules to be
completed in Semester 2. In addition during the second semester an Honours research project was
undertaken.
Academic results:
Summary of Honours research project:
Title: Alterations in SDF-1 and the CXCR4 receptor influence oral carcinoma cells in vitro.
Background: Head and Neck cancers encompass malignancies of the oral cavity, oropharynx,
hypopharynx, nasopharynx and larynx (AIgiris 2008). Approximately 95% are squamous cell
carcinomas and are associated with consumption of alcohol and tobacco use. In the UK oral cancer
is about 3% of all malignancies however there appears to be a rising incidence of these tumours
particularly amongst younger individuals. Despite recent advances in therapeutic strategies over the
last decade the five year survival for all patients with oral cancers has remained at approximately
50%.
Squamous cell carcinomas of the oral cavity have a propensity to invade locally and spread to
regional lymph nodes. There is a high rate of loco-regional recurrence and the development of a
second primary usually considered to have arisen in a field of cancerisation in an unstable oral
mucosa. In the early development of oral carcinoma it is thought that intercellular bridges or
junctional complexes between the squamous epithelial cells start to disintegrate. Cadherins,
proteins involved in the junctional complexes have been shown to be altered during tumorigenesis
(Jeanes 2008). Breakdown in intercellular communications and signalling between cells and the
extracellular matrix is thought to promote epithelial-mesenchymal transition which in turns leads to
migration and invasion of cancer cells. During invasion the extracellular matrix surrounding the
tumour cells is degraded by proteins called metalloproteinases. Tumour cells dissociated from each
other by the activity of metalloproteins can readily spread and metastase (Allinen 2004). Recent
studies have shown that some MMPs may have prognostic significance in some oral cancers (Oliveira
2011). In particular, MMP-9 which is a known downstream target of chemokine receptor 4 (CXCR4)mediated signalling. CXCR4 expressing tumour cells appear to have a more invasive phenotype and
are found in metastatic oral carcinomas (Yu 2011)
Recent research suggests there is an important role played by stromal cells during epithelialmesenchymal transition and invasion of malignant epithelial cells. Fibroblasts present in connective
tissue secrete numerous growth factors that are known to promote the proliferation and survival of
carcinoma cells in an autocrine and paracrine manner (Bhowmick 2005). Heterotypic interactions
and signals between cancer cells and tumour stromal fibroblasts stimulate migration of both cell
types towards each other, modifying the adjacent ECM and basement membrane components,
leading to breakdown of normal tissue boundaries (De Wever 2003). Important growth factors
which help to construct a stroma-dependent ‘metastasis framework’ are Transforming growth factor
beta (TGFbeta) and epidermal growth factor (EGF) (Powell 1999).
During tumorigenesis it is thought that TGFbeta and EGF stimulate processes such as angiogenesis,
escape from immunosurveillance and the appearance of tumour myofibroblasts; cells which share
characteristics of both fibroblasts and smooth muscle cells. In oral squamous carcinomas
myofibroblasts are found in the stroma adjacent to invading islands of tumour generating an image
of these cancers as the’ wound that does not heal’ (Dvorak 1986). Myofibtoblasts adjacent to
invading tumour have been found to release stromal cell-derived factor 1 (SDF-1) which has a
mitogenic effect on primary human keratinocytes and is thought to be important in promoting
metastasis of oral cancers (Florin 2005).
Stromal cell-derived factor 1 (SDF-1), also known as CXCL12, exists in two forms through alternate
splicing of a gene located on chromosome 10. It binds to the receptor CXCR4 to exert its cellular
effects (De La Luz Sierra 2004). SDF-1 has been found to be important in the development of lymph
node and distant metastases of several types of cancer including colon, breast , ovary , prostate,
lung and thyroid malignancies (Uchida 2004). The presence in tissues of the receptor for SFD-1 has
been reported to be associated with worse prognosis, with a 5-year survival of 56.8% for a CXCR4postive cohort compared with 83.3% for a CXCR4-negative group (Almofti 2004).
Aims of the project were to:
investigate the expression of CXCR4 in multiple oral carcinoma cell lines in order to identify
specific cell lines which had high and low expressions of the receptor
investigate the treatment of SDF1/CXCL12 on the proliferation of oral carcinoma cell lines
which highly expressed CXCR4.
determine the effects of SDF-1 on total MMP activity and specifically MMP-9 by oral
carcinoma cell lines
determine the effects of SDF-1 on MMP-9 expression in oral carcinoma cell lines
Methods: Five oral cancer cell lines (OSCCs) were established and used in the study. Cells were
maintained in standard keratinocyte growth medium (SKCM).
Cell Line
H357
Description
Established from a squamous cell carcinoma of the tongue from a 74
year-old male.
BICR6
Adherent keratinocyte cell line derived from a squamous cell carcinoma
of the hypopharynx of a Caucasian male.
PE/CA
Established from tongue tissue of a 45 year old male with oral squamous
cell carcinoma.
SCC15
Established from tongue tissue of a 55 year old male with squamous cell
carcinoma.
C1
Established from squamous cell carcinoma of the tongue.
Table 1: Description of OSCC cell lines
In this project real-time PCR (RQ-PCR) was used to analyze the expression of the CXCR4 and MMP9
genes. Total RNA was extracted from cell lines using a standard phenol: chloroform protocol
(TriZOL®). This total RNA was reverse transcribed to cDNA which was subsequently used for RQ-PCR
amplification. To evaluate the expression of the CXCR4 gene in the OSCC cell lines, a Taqman® based
assay was used. This involved the amplification of the cDNA by CXCR4 specific primers and a
concurrent release of a fluorescently labelled probe, where the change in the reaction fluorescence
is directly proportional to the amplification of the gene. The number of PCR cycles required for the
fluorescence in the reaction to reach a preset threshold is measured (Ct). In a similar reaction, the
same amount of cDNA template is amplified using primers specific to a stably expressed RNA, for
example 18S ribosomal RNA or β-actin. This allows normalization of the cDNA template amount
between cell lines or treatments. The expression of MMP9 was analysed in a similar fashion, with
the exception that the fluorescent probe was omitted and a fluorescent DNA intercalator is added.
The effect of increasing concentrations of SDF-1 on Total MMP production was assessed by FRET
analysis of supernatants and cell lysates. Additionally the SensoLyte Plus® 520 MMP-9 assay kit was
used to specifically assess the MMP-9 activity of the cells. All data was analysed using Excel and
Graphpad Prism software.
Results: This project analysed the expression of the CXCR4 gene in untreated oral squamous cell
carcinoma (OSCC) cell lines. All five OSCC cell lines analysed expressed CXCR4, but the levels of
expression were variable between cell lines, with the cell line BICR6 showing the greatest level of
expression. This cell line was later treated with various concentrations of the cytokine SDF-1, the
total RNA was extracted after 72 hours of treatment and subjected to RQ-PCR analysis for MMP-9
expression. This showed a dose-dependent upregulation of MMP-9, where treatment with 100
ng/ml SDF-1 increased its expression approximately four fold.
PCR for expression of CXCR4
BICR6
C1
H357
PECA
SCC15
Data 2
30
CXCR4 expression
CT
20
10
Cell lines
Figure 1: Expression by PCR of CXCR4 by OSCC cell lines
The expression of the CXCR4 receptor, as detected by qPCR was highest in BICR6 cells.
C
15
SC
PE
C
A
35
7
C
1
H
B
IC
R
6
0
BICR6 Cell Proliferation Assay
250
0 ng/ul
1ng/ul
10 ng/ul
100 ng/ul
Fluoroscence Units (x1000)
200
150
100
50
0
0
24
48
72
Time Point
Figure 2: Luminescence recorded in 96-well plates with varying concentrations of
SDF-1, at time points 0, 24, 48 and 72 hours
MMP Activity Assays
Lysates
50
0
RFU/min
10
20
30
40
50
-50
60
70
80
Lysate
Lysate
Lysate
Lysate
-100
-150
Time (min)
-200
Figure 3: Graph showing total expression of MMPs at differing
concentrations of SDF-1 in BICR6 cell lysates
Control
1ng/ml
10ng/ml
100ng/ml
Supernatants
700
S/N Control
S/N 1 ng/ml
S/N 10 ng/ml
S/N 100 ng/ml
600
RFU/min
500
400
300
200
100
0
0
10
20
30
40
50
60
70
80
Time (min)
Figure 4: Graph showing total expression of MMPs at differing
concentrations of SDF-1 in SKG media used to grow BICR6 cells
Activated MMPs
400
200
0
20
40
Time (mins)
-200
60
80
Lysates 0ng/ml
Lysates 1ng/ml
Lysates 10ng/ml
Lysates 100ng/ml
-400
Figure 5: Total expression of activated MMPs in BICR6 cell lysates treated with SKG media
containing differing concentrations of SDF-1
3000
2000
Supernatants 0ng/ml
Supernatants 1ng/ml
Supernatants 10ng/ml
Supernatants 100ng/ml
1000
0
20
40
60
80
time (mins)
-1000
Figure 6: Total expression of activated MMPs in media used to grow BICR6 cells treated with
SKG media containing differing concentrations of SDF-1
SDF-1, over the concentration 1-100ng/ml had no significant effect on total MMP activity.
MMP9
4.5
4
3.5
3
2.5
2
1.5
1
0.5
0
0
1
10
100
Figure 7: Graph of MMP-9 expression at different concentrations of SDF-1
Q-PCR analysis of MMP-9 mRNA expression in BICR6 cells showed a fold increase in expression
following incubation with SDF-1 at a concentration of 100ng/ml.
Discussion and conclusions: CXCR4, the receptor for the CXCL12 ligand, has been associated with an
increased risk of invasion and a worse prognosis in several cancers. Several OSCC cell lines were
investigated to detect and quantify the expression of the CXCR4 gene. The cell line with highest
expression was used in the main study. Q-PCR was chosen as the method of analysis over traditional
standard PCR due to its enhanced precision in determining gene expression, compared to standard
PCR. The results of the cell proliferation assay show that SDF-1 treatment caused an increase in the
proliferation of BICR6 cells after 72 hours. It demonstrates that, at 72 hours, this increase is
proportional to the concentration of SDF-1 used (up to 100ng/ml). Previous studies have supported
the claim that CXCR4 expression enhances the ability of a tumour to proliferate and have suggested
the CXCR4 as a potentially valuable biomarker to predict the progression of OSCC (Xu 2006) In OSCC,
knockdown of CXCR4 genes leads to the inhibition of growth and invasiveness (Hong 2009)leading to
the belief that the receptor plays a pivotal role in the proliferation of tumour cells and thus the
progression of the cancer.
The total MMP activity assay measured activity both inside the cell and secreted into the media. The
aim was to determine if addition of SDF-1 to the media had an effect. Initial results from the activity
assay showed that the levels of expression were quite low. This suggested that the proteases were
secreted, if at all, in a mostly inactive form. For comparison, the samples were activated using the
APMA mercurial compound, and the assay was repeated. Following APMA application higher levels
of total activity were detected. This is consistent with studies that demonstrated how MMPs are
initially synthesised as inactive zymogens, with a pro-peptide domain that must be removed before
the enzyme is active. The pro-peptide domain contains a conserved cysteine residue (known as a
“cysteine switch”) which interacts with the zinc in the active site and prevents binding and cleavage
of the substrate, keeping the enzyme in an inactive form Pei 2000. Overall, the total MMP activity
does not appear to have been influenced by the concentration of SDF-1 in the media. This is in
contrast to studies in other cancer types which show an increased MMP activity related to the
influence of SDF-1 in several cancer models (Tan 2008; Tang 2008). MMP-9, also known as
Gelatinase-B, has been implicated in the progression of several cancers including prostate
carcinoma, as well as leukaemia and lymphoma {{161 Suh,J. 2004}}. Several studies have
demonstrated how it has been associated with increased tumour aggressiveness and regional
invasion, both in OSCC and in HNSCC in general. .
Overall, this project achieved the proposed aims. The project showed how the treatment of OSCC
cells with SDF-1 can have an enhancing effect on their proliferation. MMP activity assays were used
to study total MMP and MMP-9 activity in OSCC cell cultures. Data indicated that almost all MMPs
are released by cells into the culture supernatant, with minimal activity remaining in the cytosol.
Moreover the vast majority of secreted MMP is in an inactive form requiring activation postsecretion. While SDF-1, at a concentration of 100ng/ml, stimulated MMP-9 mRNA expression by the
OSCC cells, this effect was not seen at an activity level.
References
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008
17;371(9625):1695-709.
Almofti, A., Uchida, D., Begum, N.M., Tomizuka, Y., Iga, H., Yoshida, H. & Sato, M. 2004, "The
clinicopathological significance of the expression of CXCR4 protein in oral squamous cell carcinoma",
International journal of oncology, vol. 25, no. 1, pp. 65-71
De La Luz Sierra, M., Yang, F., Narazaki, M., Salvucci, O., Davis, D., Yarchoan, R., Zhang, H.H., Fales, H.
& Tosato, G. 2004, "Differential processing of stromal-derived factor-1alpha and stromal-derived
factor-1beta explains functional diversity", Blood, vol. 103, no. 7, pp. 2452-2459.
De Wever, O., Demetter, P., Mareel, M. & Bracke, M. 2008, "Stromal myofibroblasts are drivers of
invasive cancer growth", International journal of cancer.Journal international du cancer, vol. 123, no.
10, pp. 2229-2238.
Dvorak, H.F. 1986, "Tumors: wounds that do not heal. Similarities between tumor stroma generation
and wound healing", The New England journal of medicine, vol. 315, no. 26, pp. 1650-1659.
Florin, L., Maas-Szabowski, N., Werner, S., Szabowski, A. & Angel, P. 2005, "Increased keratinocyte
proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts", Journal of cell science,
vol. 118, no. Pt 9, pp. 1981-1989.
Jeanes, A., Gottardi, C.J. & Yap, A.S. 2008, "Cadherins and cancer: how does cadherin dysfunction
promote tumor progression?", Oncogene, vol. 27, no. 55, pp. 6920-6929
Oliveira, L.R. & Ribeiro-Silva, A. 2011, "Prognostic significance of immunohistochemical biomarkers
in oral squamous cell carcinoma", International journal of oral and maxillofacial surgery, vol. 40, no.
3, pp. 298-307.
Pei, D., Kang, T. & Qi, H. 2000, "Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a
type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion
and activation", The Journal of biological chemistry, vol. 275, no. 43, pp. 33988-33997.
Powell, D.W., Mifflin, R.C., Valentich, J.D., Crowe, S.E., Saada, J.I. & West, A.B. 1999, "Myofibroblasts.
I. Paracrine cells important in health and disease", The American Journal of Physiology, vol. 277, no.
1 Pt 1, pp. C1-9.
Suh, J. & Rabson, A.B. 2004, "NF-kappaB activation in human prostate cancer: important mediator or
epiphenomenon?", Journal of cellular biochemistry, vol. 91, no. 1, pp. 100-117
Tan, C.T., Chu, C.Y., Lu, Y.C., Chang, C.C., Lin, B.R., Wu, H.H., Liu, H.L., Cha, S.T., Prakash, E., Ko, J.Y. &
Kuo, M.L. 2008, "CXCL12/CXCR4 promotes laryngeal and hypopharyngeal squamous cell carcinoma
metastasis through MMP-13-dependent invasion via the ERK1/2/AP-1 pathway", Carcinogenesis, vol.
29, no. 8, pp. 1519-1527.
Tang, C.H., Tan, T.W., Fu, W.M. & Yang, R.S. 2008, "Involvement of matrix metalloproteinase-9 in
stromal cell-derived factor-1/CXCR4 pathway of lung cancer metastasis", Carcinogenesis, vol. 29, no.
1, pp. 35-43.
Uchida, D., Begum, N.M., Tomizuka, Y., Bando, T., Almofti, A., Yoshida, H. & Sato, M. 2004,
"Acquisition of lymph node, but not distant metastatic potentials, by the overexpression of CXCR4 in
human oral squamous cell carcinoma", Laboratory investigation; a journal of technical methods and
pathology, vol. 84, no. 12, pp. 1538-1546.
Xu, M., Li, W.Y., Chen, X.M., Wang, X.H., Huang, J. & Wang, L. 2006, "Investigation of CXCR4 in oral
squamous cell carcinoma and its influence on proliferation of tumor cells in vitro]", Zhonghua kou
qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = Chinese journal of stomatology, vol. 41, no. 6,
pp. 372-375.
Yu, T., Wu, Y., Helman, J.I., Wen, Y., Wang, C. & Li, L. 2011, "CXCR4 promotes oral squamous cell
carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK
signaling pathway", Molecular cancer research : MCR, vol. 9, no. 2, pp. 161-172.
Chris Gault 2012