Nanotechnology (elective) VII Sem UNIT 2: PREPARATION OF
advertisement

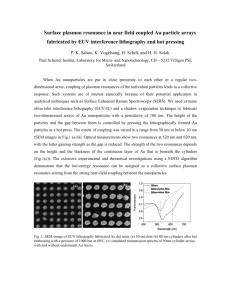
Nanotechnology (elective) VII Sem UNIT 2: PREPARATION OF NANOMATERIALS Preparation of nanomaterials: Bottom-up and Top-down approaches with examples. Physical methods: Ball milling, Inert gas condensation, Arc discharge, Ion sputtering, Laser ablation, Spray pyrolysis, Flame pyrolysis, Thermal evaporation, Pulsed laser deposition, Molecular beam epitaxy. Chemical methods: Metal nanocrystals by reduction, Solvothermal synthesis, Photochemical synthesis, Electrochemical synthesis, Micelles and Microemulsions, Chemical vapour deposition (CVD), Sol -gel process. Lithographic techniques: Photolithography, Electron beam and Focused ion beam lithography. [Brief explanation for each] 8 Hrs. 1. Bottom-up and Top-down approaches 1.1. Bottom-up approach 1.2. Top-down approach 1.3. Advantage and disadvantages of bottom-up and top-down approaches 2. Preparation of nanomaterials 2.1. Physical methods 2.1.1. Ball milling 2.1.2. Inert gas condensation 2.1.3. Arc discharge 2.1.4. Ion sputtering 2.1.5. Laser ablation 2.1.6. Spray pyrolysis 2.1.7. Flame pyrolysis 2.1.8. Thermal evaporation 2.1.9. Pulsed laser deposition 2.1.10 Molecular beam epitaxy 2.2. Chemical methods 2.2.1. Micelles and Microemulsions 2.2.2. Chemical vapour deposition (CVD) 2.2.3. Sol-gel process 2.3 Lithography 2.3.1. Photolithography 2.3.2. Particle beam lithography: Electron beam and Focused ion beam lithography Model questions 1 Nanotechnology Unit 2: Preparation of nanomaterials 1. BOTTOM-UP AND TOP-DOWN APPROACHES Using the nanotechnology, we can arrange atoms and molecules exactly as we want. In nano technology, manufactured products are made from atoms. The properties of these products depend on how the atoms are arranged. For example, if we rearrange the atoms in coal, we get diamond. If we rearrange the atoms in sand (and add a pinch of impurities), we get computer chips. Manipulation and observation of nanostructures is possible due to the combination of analytical tools e.g. Atomic Force Microscope (AFM), and Scanning Tunnelling Microscope (STM), and instruments for refined processes (e.g. Electron Beam Lithography and Molecular Beam Epitaxy). Two main approaches are used for the synthesis of nanomaterials and the fabrications of nanostructures in nanotechnology. They are: a) Bottom-up approach b) Top-down approach The difference between nanotechnology and conventional technology is that, the bottom-up approach is preferred in nanotechnology, where as conventional technology usually use the topdown approach. The difference between two approaches can be explained simply by using a example of powder production, where the chemical synthesis represents the bottom-up approach while the crushing and milling of chunks represents the equivalent top-down process. An illustration of the top-down approach vs. the bottom-up approach is shown in Figure 1. Fig. 1: The top-down approach vs. the bottom-up approach. 1.1. BOTTOM-UP APPROACH Bottom-up approach is often emphasized in nanotechnology literature, though bottom-up is nothing new in materials synthesis. Bottom-up approach refers to the construction of nanomaterial from the bottom, i.e. atom-by-atom, molecule-by-molecule or cluster-by-cluster. 2 Nanotechnology Unit 2: Preparation of nanomaterials In bottom-up approach, the atoms or molecules are used as the building blocks to produce nanoparticles, nanotubes, nanorods, thin films or layered structures. According to their dimensionality, these features are also referred to as zero-, one- or two-dimensional nanostructures. Following figure also demonstrates the building of particles, layers, nanotubes or nanorods from atoms (ions) or molecules. Fig. 2: Bottom-up approach result in particle, nanotube, nanorods, thin films or layers structures. Examples for Bottom-up approach: 1. Synthesis of large polymer molecule is a typical example for bottom-up approach, in which individual building blocks (i.e. monomers) are assembled to a large molecule or polymerized into bulk material. 2. Fabrication of integrated circuits (assembling different components, e.g. resistors, capacitors, transistors etc) on a PCB. 3. Crystal growth, where growth species either atoms, or ions or molecules orderly assemble into desired crystal structure on the growth surface. 4. Colloidal dispersion in the synthesis of nanoparticles. 5. Nanolithography and nano-manipulation. 6. Production of salt and nitrate in chemical industry, the growth of single crystals and deposition of films in electronic industry. Although such processes provide tremendous freedom among the resultant products, the number of possible structures to be obtained is comparatively small. In order to obtain the ordered structures, bottom-up processes must be supplemented by the self-organization of individual particles, in which the atoms or molecules arrange themselves into a structure due to their natural properties. Crystals grown for the semiconductor industry provide an example of self assembly, as does chemical synthesis of large molecules. 1.2. TOP-DOWN APPROACH The word "top-down" means starting from large pieces of material and producing the intended structure by mechanical or chemical methods. This situation is shown schematically in figure. As long as the structure are within a range of sizes that are accessible by either mechanical tools or photolithography processes, then top-down processes have an unmatched flexibility in their application. Top-down is in general an extension of lithography. 3 Nanotechnology Unit 2: Preparation of nanomaterials The principle behind the top-down approach is to take a bulk piece of the material and then modify it into the wanted nanostructure. Cutting, grinding and etching are typical fabrication techniques, which have been developed to work on the nano scale. The sizes of the nanostructures, which can be produced with top-down techniques, are between 10 to 100 nm. Fig. 3: The intended product is obtained by the application of mechanical or chemical processes. Examples: 1. Creating circuits on the surface of a silicon microchip by etching. 2. Attrition or milling in making nanoparticles, NOTE: Lithography may be considered as a hybrid approach, since the growth of thin films is bottom-up whereas etching is top-down. 1.3. ADVANTAGE AND DISADVANTAGES OF BOTTOM-UP & TOP-DOWN APPROACHES Both approaches play very important roles in modern industry and most likely in nanotechnology as well. There are advantages and disadvantages in both approaches. An advantage of the bottom-up approach is the better possibilities to obtain nanostructures with less defects and more homogeneous chemical compositions. This is due the mechanisms utilized in the synthesis of nanostructures reducing the Gibbs free energy, so that the produced nanostructures are in a state closer to a thermodynamic equilibrium. In bottom-up approach, for most materials, there is no difference in physical properties of materials regardless of the synthesis routes, provided that chemical composition, crystallinity, and microstructure of the material in question are identical. Of course, different synthesis and processing approaches often result in appreciable differences in chemical composition, crystallinity, and microstructure of the material due to kinetic reasons. Consequently, the material exhibits different physical properties. Bottom-up approach also promises a better chance to obtain nanostructures with less defects, more homogeneous chemical composition, and better short and long range ordering. This is because the bottom-up approach is driven mainly by the reduction of Gibbs free energy, so that nanostructures and nanomaterials produced are in a state closer to a thermodynamic equilibrium state. On the contrary, top-down approach most likely introduces internal stress, in addition to surface defects and contaminations. Top-down approach has proven unsuccessful for several purposes. One of the problems with the top-down approach is the imperfection of the surface structure. Such defects in the surface structure can have a significant impact on physical properties and surface chemistry of the nanostructure, since the surface area to volume ratio in nanostructures and nanomaterials is very large. The surface imperfection would result in a reduced conductivity due to inelastic surface 4 Nanotechnology Unit 2: Preparation of nanomaterials scattering, which in turn would lead to the generation of excessive heat and thus impose extra challenges to the device design and fabrication. Even though there are problems connected to using a top-down approach, this is the method of choice when highly complex structures are made. This is the case in the integrated circuit industry, where nano-sized structures are cut in plain silica wafers using laser techniques. 2. PREPARION OF NANOMATERIALS There are physical as well as chemical methods to prepare nanomaterials. However, the most powerful methods are the chemical methods. It can be seen from the following that, for each type, there is a large number of possibilities. The technique to be used depends on material of interest, type of nanostructures [zero dimensional (0-D), one dimensional (1-D) or two dimensional (2-D) material], size, quantity etc. 1. Physical methods: Ball milling, Inert gas condensation, Arc discharge, Ion sputtering, Laser ablation, Spray pyrolysis, Flame pyrolysis, Thermal evaporation, Pulsed laser deposition, Molecular beam epitaxy. 2. Chemical methods: Metal nanocrystals by reduction, Solvothermal synthesis, Photochemical synthesis, Electrochemical synthesis, Micelles and Microemulsions, Chemical vapour deposition (CVD), Sol-gel process. 3. Lithographic techniques: Photolithography, Electron beam and Focused ion beam lithography. 2.1. PHYSICAL METHODS 2.1.1. BALL MILLING It is one of the simplest ways of making nanoparticles of some metals and alloys in the form of powder. There are many types of ball mills viz. planetary, vibratory, rod, tumbler etc. A ball mill (a type of grinder) is a cylindrical device used for grinding (or mixing) materials to as small as few nanometers. A ball mill consists of a cylindrical capped container that sits on two drive shafts (pulleys and belts are used for rotary motion) or directly connected to motor for rotation. Size of container, of course, depends upon the quantity of interest. The container is partially filled with the material to be ground (powder or flakes) plus the grinding medium (hard spherical balls). Initial material can be of arbitrary size and shape. Different materials are used as grinding media, including tungsten carbide balls, ceramic balls, flint pebbles and stainless steel balls. Larger balls used for milling, produce smaller grain size and larger defects in the particles. Usually 2:1 mass ratio of balls to material is advisable. If the container is more than half filled, the efficiency of milling is reduced. When the container is rotating about a horizontal axis, the material is forced to the walls and is pressed against the walls. This internal cascading effect reduces the material to a fine powder. By controlling the speed of rotation of the container as well as duration of milling, it is possible to ground the material to fine powder (few nm to few tens of nm) whose size can be quite uniform. This process may add some impurities from balls. The container may be filled with air or inert gas. However, this can be an additional source of impurity, if proper precaution to use high purity gases is not taken. 5 Nanotechnology Fig. 4: Common ball mill Unit 2: Preparation of nanomaterials Fig. 5: Planetary ball mill Some of the materials like Co, Cr, W, Ni- Ti, Al-Fe, Ag-Fe etc are made nanocrystalline using ball mill. Few milligrams to several kilograms of nanoparticles can be synthesized in a short time of a few minutes to a few hours. PLANETARY BALL MILL: Apart from common ball mills (Fig. 4) there is a second type of ball mill called planetary ball mill (Fig. 5). Planetary ball mills are mainly used in laboratories for grinding sample material down to very small sizes. A planetary ball mill consists of at least one grinding jar which is arranged eccentrically on a so-called sun wheel. The direction of movement of the sun wheel is opposite to that of the grinding jars (ratio: 1:2 or 1:1 or else). The grinding balls in the grinding jars are subjected to superimposed rotational movements, the so-called coriolis forces. The difference in speeds between the balls and grinding jars produces an interaction between frictional and impact forces, which releases high dynamic energies. The interplay between these forces produces the high and very effective degree of size reduction of the planetary ball mill. Figure 5 shows schematic of a ball mill in planetary motion. Sketch shows the material thrown against the wall during the course of rotation of a single container. Dark regions are illustrating the powder material, while the rest is empty. NOTE: Key properties of grinding media are size, density, hardness, and composition. Size: The larger the media particles, the smaller the particle size of the final product. At the same time, the grinding media particles should be substantially larger than the largest pieces of material to be ground. Density: The grinding media should be denser than the material to be ground. It becomes a problem if the grinding media floats on top of the material to be ground. Hardness: The grinding media needs to be durable enough to grind the material, but where possible should not be so tough that it also wears down the tumbler at a fast pace. Composition: Various grinding applications have special requirements. Some of these requirements are based on the fact that some of the grinding media will be in the finished product. Others are based in how the media will react with the material being ground. 2.1.2. INERT GAS CONDENSATION (IGC) The inert gas condensation (IGC) process is one of the most known and simplest technique for production of nanoparticles, in particular, metal nanopowders. In its basic form, the method consists of evaporating a material (e.g.: a metal) in a chamber using resistive heating. This chamber has been previously evacuated to about 10-7 torr and backfilled with inert gas (e.g. helium gas) to a low-pressure. 6 Nanotechnology Unit 2: Preparation of nanomaterials The metal vapor cools through collisions with the inert gas atoms. Thus, metal vapour losses their kinetic energy, becomes supersaturated and then nucleates homogeneously. The particle size is usually in the range 1-100 nm and can be controlled by varying the inert gas pressure. Ultimately, the particles are collected and may be compacted to produce a dense nanomaterial. Fig. 6: Schematic lay out of a typical inert gas condensation system. 2.1.3. ARC DISCHARGE This is one of the simplest and useful method which leads to mass scale production of fullerens and carbon nanotubes. The arc discharge set up is as shown in figure. Basically, it requires water cooled vacuum chamber and electrodes to strike an arc between them. The positive electrode itself acts as the source of material. If some catalysts are to be used, there can be some additional thermal source of evaporation. Inert gas or reactive gas introduction is necessary. Fig. 7: Schematic lay out of a typical arc discharge system. 7 Nanotechnology Unit 2: Preparation of nanomaterials Usually the gap between the electrodes is ~ 1 mm and high current ~ 50 to 100 amperes is passed from a low voltage power supply (12-15 volts). Inert gas pressure is maintained in the vacuum system. When an arc is set up, anode material evaporates. This is possible as long as the discharge can be maintained. The adjustment of the electrode gap without breaking the vacuum becomes essential, as one of the electrode burns and gap increases. 2.1.4. ION SPUTTERING Sputtering is a process in which atoms are ejected from a solid target material due to bombardment of the target by energetic particles. Sputtering process is commonly utilized for thinfilm deposition. It is a very good technique to deposit multilayer films for mirrors or magnetic films for spintronics. In sputter deposition, some inert gas ions (e.g. argon) are incident on a target at a high energy. Target material may be some alloy, ceramic or compound. Depending on energy of the ions and ratio of ion mass to target atoms mass, the ion-target interaction can be a complex phenomenon. The ions become neutral at the surface but due to their energy the incident ions may get implanted, get bounced back, create collision cascades in target atoms, displace some of the atoms in the target creating vacancies, interstitials and other defects, desorb some adsorbates, create photons while losing energy to target atoms or even sputter out some target atoms/molecules, clusters, ions and secondary electrons. Fig. 8 shows a schematic picture of various possibilities. Fig. 8: Interaction of an ion with the target Sputter deposition can be carried out using Direct Current (DC) sputtering, Radio Frequency (RF) sputtering or magnetron sputtering. In all these methods, one uses discharge or plasma of some inert gas atoms or reactive gases. The deposition is carried out in a required gas pressurized high vacuum or ultra high vacuum system equipped with two electrodes (one is a sputter target and the other is a substrate) and gas introduction facility etc. Although the system during deposition is at high gas pressure, low base pressure ensures that the adequate purity is obtained if high purity gases are used. DC SPUTTERING: Target and substrate serve as electrodes and face each other in a typical sputtering chamber. Target is held at high negative voltage [i.e. acts as cathode: conductive material, e.g. metal] and substrate may be at positive voltage or ground [i.e. acts as anode] (Fig. 9). Substrates may be simultaneously heated or cooled depending upon the requirement. Once the required base pressure is attained in the vacuum system, an inert gas (e.g. argon gas) is introduced into the system at a pressure < 0.1 torr as the medium to initiate and maintain a discharge. When an electric field of several kilovolts per centimeter is applied between the electrodes, a glow discharge is set up with different regions such as cathode glow, Crooke's dark space, negative 8 Nanotechnology Unit 2: Preparation of nanomaterials glow, Faraday dark space, positive column, anode dark space and anode glow. These regions are the result of plasma, i.e. a mixture of free electrons, ions, neutrals and photons released in various collisions. The density of various particles and the length over which they are spread and distributed depends upon the gas pressure. The free electrons are accelerated by the electric fiel, and gain sufficient energy to ionize argon atoms. If the gas density or pressure is too low, then the electrons will simply strike the anode without having gas phase collision with argon atoms. However, if the gas density or pressure is too high, then the electrons will not gain sufficient energy when they strike gas atoms to cause ionization. The positive ions, Ar+ ions, produced in the discharge strike the cathode (the source target) resulting in the ejection of neutral target atoms through momentum transfer. These ejected atoms move towards the opposite electrode (anode: substrate) and deposit there. Thus, sufficiently large number of Ar + ions are generated that can be used to sputter the target. Fig. 9: Schematic layout of a typical DC sputtering unit. RF SPUTTERING: If the target to be sputtered is insulating, it is difficult to use DC sputtering. This is because it would mean the use of exceptionally high voltage (> 10 6 V) to sustain a discharge between the electrodes. Such high voltage will harm the target source and produced film. In DC sputtering, 100 to 3000 volts is a usual voltage. However if some high frequency lower voltage is applied, the cathode and anode alternatively keep on changing their polarity and oscillating electrons cause sufficient ionization. In principle, 5 to 30 MHz frequency can be used and the electrodes can be insulating. However, 13.56 MHz is a commonly used frequency for deposition, as it is reserved worldwide for this purpose and others are available for communication. Target itself biases to negative potential becoming cathode when the arrangement as depicted in Fig. 10 is used. The capacitor in the circuit will have low RF impedance and will allow the formation of a DC bias on the electrodes Fig. 10: Schematic layout of a typical AC sputtering unit. 9 Nanotechnology MAGNETRON SPUTTERING Unit 2: Preparation of nanomaterials RF or DC sputtering efficiency can be further increased by using magnetic field. By adding a magnetic field in the system, the electron will move in spiral path. This will hugely increase the ionization density of argon gas and opportunity of collision. The density will be 1010 ion/cm3 and increase to 1013 ion/cm3. Therefore, the deposition rate can be increased. When a charged particle (Q) moves in a region where both electric and magnetic fields are present, then the force acting on it is given by Lorentz force equation. F =QE +Q (u×B) Where, u is a velocity vector. If E and B are parallel to each other as illustrated in Fig. 11 and an electron leaves the cathode at angle θ = 0°, then u × B = 0 and only electric field vector acts on an electron. However, an electron making an angle θ > 0°, would have both electric and magnetic fields acting on it. The magnitude of velocity component in the direction of electric field would be "Q u cos θ ". The component due to the magnetic field would be perpendicular to both u and B with a magnitude "Q B u sin θ ". Fig. 11: Effect of E and B on electron. As soon as electron enters into the magnetic field, it starts moving in a circular path (after experiencing magnetic force). Here, magnetic force (Fm) balances centripetal force [m(usinθ)2/r: experienced by a charged particle] while circulating in an orbit of radius "r". ⇒ Fm = m usinθ) 2 Where, "u" is the velocity of a charged particle moving in a circular path of radius "r". r 2 ⇒ 2 2 BQusinθ = mu sin θ ⇒ r r= musinθ BQ [∵ Fm = B Q u sinθ ] Electron moves in a helical path and is able to ionize more atoms in the gas. In practice, both parallel and perpendicular magnetic fields to the direction of electric field are used to further increase the ionization of the gas, increasing the efficiency of sputtering. By introducing gases like O2, N2, NH3, CH4, H2S etc. while metal targets are sputtered, one can obtain metal oxides like A1 2O3, nitrides like TiN, carbides like WC etc. This is known as "reactive sputtering". NOTE: Creating plasma: The terms glow discharge and plasma are often used to mean the same thing. Plasma is a mixture of free electrons, ions and photons. Plasma in overall is neutral but there can be regions, which are predominantly f positive or negative charges. One can get plasma in different gases at different pressures by using DC or AC. Different regions of plasma generated by applying a high DC voltage can be divided into five regions as: Fig. 12: (a) Generation of plasma by applying high voltage between two electrodes in an evacuated glass tube. (b) Different regions of plasma. (i) Cathode or Crook's dark space (ii) Negative space glow (iii) Faraday's dark space (iv) Positive column (v) Anode dark space 10 Nanotechnology Unit 2: Preparation of nanomaterials The extent of these regions depends upon the pressure of the gas. The glow discharge in a glass tube with two electrodes at the end filled with air has following characteristics at different pressures. Pressure (torr) Appearance of discharge ~ 10 ~ 1-0.5 ~ 0.5 ~ 0.3 ~ 0.1 ~ 0.05 ~ 0.005 General glow discharge Closely spaced striations in positive column 10 mm spacing of striations Crook's dark space 5 mm long Crook's dark space 10 mm long Crook's dark space 20 mm long Discharge disappearance 2.1.5. LASER ABLATION Laser ablation is the process of removing material from a solid surface (or occasionally liquid) by irradiating it with a laser beam. The basic schematic layout of laser ablation system is as shown in the figure 13. Essentially it consists of a high vacuum or ultra high vacuum (UHV) system filled with inert gas or reactive gas, laser beam, solid target and cooled substrate. Usually laser giving UV wavelength such as eximer laser (see Table 1) is necessary because lasers with other wavelengths like IR or visible often reflected by some of the metal surfaces. Table 1: Wavelengths of some commonly used excimer lasers Fig. 13: Schematic layout of laser ablation system. In a typical procedure, atoms from solid surface of the target are evaporated by making powerful laser beam to fall on the surface of the target. These atoms collide with inert gas atoms (or reactive gases) forming clusters. These clusters are condense on the cooled substrate. The method is known as laser ablation. Gas pressure in system is very critical in determining the particle size and distribution. Simultaneous evaporation of another material and mixing the two evaporated materials in inert gas leads to the formation of alloys or compounds. This method can produce some novel phases of the materials, which are not normally formed by other techniques. For example: Single wall carbon nanotubes (SWNT) are mostly synthesized by this method. 11 Nanotechnology Unit 2: Preparation of nanomaterials 2.1.6. SPRAY PYROLYSIS The word pyrolysis has originated from the Greek words "pyro" (means fire) and "lysis" (means separating). Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase of the starting material, and is irreversible. Spray pyrolysis is basically a solution process and has been widely used in the preparation of metal and metal oxide powder. The process can be simply described as converting microsized liquid droplets of precursor (starting source material) or precursor mixture into solid particles through heating. In practice, spray pyrolysis involves several steps: 1. Generating microsized droplets of liquid precursor or precursor solution 2. Evaporation of solvent 3. Condensation of solute 4. Decomposition and reaction of solute 5. Sintering (heating without melting) of solid particles Schematic of spray pyrolysis system is shown in the figure 14. Spray pyrolysis is a process in which a thin film is deposited by spraying a solution on a heated surface, where the constituent reacts to form a chemical compound. The chemical reactants are selected such that products other than the desired compound are volatile at the temperature of deposition. The process is particularly useful for the deposition of oxides and has long been a production method for a long period for applying a transparent electrical conductor of Tin oxide (SnO2) or Stannic oxide to glass. Fig. 14: Schematic layout of spray pyrolysis system. 2.1.7. FLAME PYROLYSIS The word pyrolysis has originated from the Greek words "pyro" (means fire) and "lysis" (means separating). Pyrolysis is a thermochemical decomposition of organic material at elevated 12 Nanotechnology Unit 2: Preparation of nanomaterials temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase of the starting material, and is irreversible. Flame pyrolysis or thermolysis is a chemical decomposition of chemical compound caused by heat. Flame spray pyrolysis can be used to produce a wide array of high purity nanopowders ranging from single metal oxides such as alumina to more complex mixed oxides, metals and catalysts. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway, which avoids a possible explosion. Schematic of flame pyrolysis system is as shown in figure 15. Flame pyrolysis is a gas phase synthesis method, where flame heat is used to vaporize precursor material and initiate the chemical reactions to produce nano-particles. Flame spray pyrolysis is a one step process in which a liquid feed (a metal precursor(s) dissolved in a solvent) is sprayed with an oxidising gas into a flame zone. The spray is combusted and the precursor(s) are converted into nanosized metal or metal oxide particles. The technique is flexible and allows the use of a wide range of precursors, solvents and process conditions, thus providing control over particle size and composition. Fig. 15: Schematic layout of flame pyrolysis. 2.1.8. THERMAL EVAPORATION [or RESISTIVE EVAPORATION] Thermal/resistive evaporation is a proven and economical method of depositing thin film coatings in vacuum. Resistive evaporation is used in various applications ranging from decorative coating to semiconductor manufacturing processes.. Resistive thermal evaporation is one of the most commonly used metal deposition techniques. Metals such as iron, nickel, aluminum, copper, tin, silver, gold and platinum are frequently deposited using thermal evaporation methods. It consists of vaporizing a solid material (pure metal, eutectic or compound) by heating it to sufficiently high temperatures and re-condensing it onto a cooled substrate to form a thin film. As the name implies, the heating is carried out by passing a large current through a filament container (usually in the shape of a basket, boat or crucible) which has a finite electrical resistance. The choice of this filament material is dictated by the evaporation 13 Nanotechnology Unit 2: Preparation of nanomaterials temperature and its inertness to alloying/chemical reaction with the evaporant. Traditionally the filament material is made by tungsten [W: melting point=3380 °C] or thallium [Ta: M.P=2980°C) or molybdenum [Mo: M.P =2630 °C] which is with very high melting temperature. Typical layout of thermal evaporation system is as shown in the figure 16. In the thermal evaporation process, the material to be deposited (source material: metal) is loaded into a container called a crucible which is kept inside a vacuum chamber. The crucible is resistively heated by passing a high current to a temperature equal to the melting point of the source material. A high current flowing through the crucible heats it up, melts and causes evaporation of source material. These vapours of atoms travel in straight lines and they strike the surface of the substrate where they accumulate to form a film. This method is useful for depositing many layers of different materials without chemical interaction between different layers. Factors that influences the deposition thickness: 1. Pressure inside the vacuum chamber. 2. Temperature of the substrate. 3. Power flowing through the crucible. 4. Distance between source to substrate. Vacuum pump Fig. 16: Schematic layout of thermal evaporation system. Advantages: 1. Instrumentation is relatively simple and cheap. 2. Less substrate surface damage. 3. Excellent purity of films. 4. Source material (material to be deposited) can be to different shape depending on the need. Disadvantages: 1. Limited to low melting point metals, e.g.: Al, Au, Ni, Cr… 2. Fit only for metals, the dielectric material is difficult to melt and evaporate to form thin films. 3. The speed of deposition is very slow. 4. The hardness of the film is not good and the density is poor. 5. The materials that deposit on the wall of the chamber can contaminate later depositions. 6. Sometimes there are problems with film-thickness uniformity and uniform doping over largearea substrates. 14 Nanotechnology Unit 2: Preparation of nanomaterials 2.1.9. PULSED LASER DEPOSITION Pulsed laser deposition (PLD) is an efficient method to produce thin films by utilizing a technique called laser ablation. PLD can be used to deposit a wide range of materials from polymers to metals. PLD is applicable to almost any material, in particular to compounds (especially multi-element oxides) that are difficult or impossible to produce in thin-films by other techniques. Examples of such materials include complex ceramic materials such as hightemperature superconductors and certain magnetic materials [e.g. yttrium iron garnet (YIG) and ferromagnetic shape-memory (FSM) alloy Ni-Mn-Ga]. PLD was the first technique used to successfully deposit a superconducting YBa2Cu3O7 thin film. Typical layout of pulsed laser deposition (PLD) system is as shown in the figure 17. In general, the idea of PLD is simple. A high power pulsed laser beam is focused onto the surface of a solid target. The strong absorption of the electromagnetic radiation by the solid surface leads to rapid evaporation of the target material. Finally, the evaporated material get deposited onto the substrate. The evaporated materials consist of highly excited and ionized species. Fig. 17: Schematic layout of pulsed laser deposition system. Factors that influence deposition thickness: 1. Nature of target material. 2. Pulse energy of laser beam. 3. Distance between target to substrate. 4. Type of gas and pressure in chamber (e.g. oxygen, argon, etc.). Advantages of Pulsed Laser Deposition: 1. Conceptually PLD is a simple process: a laser beam vaporizes a target surface, producing a film with the same composition as the target. 2. Cost-effective: one laser can serve many vacuum systems. 3. Fast process: high quality thin films can be grown reliably in 10 or 15 minutes. 4. Films made by PLD can be extremely smooth and amorphous, or crystalline depending on the chamber atmosphere. 15 Nanotechnology 2.1.10. MOLECULAR BEAM EPITAXY (MBE) Unit 2: Preparation of nanomaterials EPITAXY: The term epitaxy is derived from the Greek words "epi" means "above" and "taxis" means "in ordered manner". So, the word epitaxy means "to arrange upon". Epitaxy is the method of depositing a thin film of single crystal material on a mono-crystalline substrate. Epitaxial films can be grown from gaseous or liquid precursors, and the substrate acts as a seed crystal. Also, orientation of the film deposited is identical to those of the substrate. The other thin-film deposition methods grow polycrystalline or amorphous films even on single-crystal substrates. If the film and substrate are same materials, then the process is called homoepitaxy or simply isoepitaxy. If the film and substrate are different materials, then the process is called heteroepitaxy. The epitaxial growth techniques are widely used for the synthesis of nanostructured materials and semiconductors. This is an affordable method of high quality crystal growth for semiconductor materials like silicon (Si), germanium (Ge), gallium nitride (GaN), gallium arsenide (GaAs) and indium phosphide (InP). There are different type of epitaxial growth techniques, such as vapour phase epitaxy, molecular beam epitaxy, liquid phase epitaxy, solid phase epitaxy and atomic layer epitaxy. MOLECULAR BEAM EPITAXY (MBE): Molecular beam epitaxy is an effective epitaxial growth technique to fabricate metal, insulator, semiconductor or superconductor epitaxial layers. This technique of deposition can be used to deposit a precise nanometre length elemental or compound quantum dots, quantum wells, quantum wires etc. in a very controlled manner. Fig. 18: Schematic layout a typical of molecular beam epitaxy system. Schematic representation of a typical MBE system is shown in figure 18. MBE essentially consists of an ultra-high vacuum chamber [UHV: ~ 10 −11 mbar] into which a substrate is loaded onto a heated sample holder. Precursors (i.e. source materials) of desired elements (e.g.: Ga, As, Al, P, In, etc.) are then loaded into heated crucibles or furnaces called Knudsen cells or effusion cells, outfitted with computer controlled shutters on their exits. The precursors are then heated such that, when the shutters are opened, one obtains a beam of atoms directed towards the substrate. Under such low pressures, the atomic species have very long mean free paths allowing them to 16 Nanotechnology Unit 2: Preparation of nanomaterials reach the substrate without collisions with other gas phase species in the chamber (i.e. geometrical size of the chamber is less than the mean free path of the particle). The substrate is sufficiently heated and frequently rotated to improve the growth homogeneity. By controlling the temperature as well as the sequence/timing of opening and closing the shutters, one can deposit uniform films of semiconductor materials. For example: In solid-source MBE, ultra-pure elements such as gallium and arsenic are heated in separate effusion cells until they begin to slowly evaporate. The evaporated elements are then condensed on the wafer, where they may react with each other to form thin film of singlecrystalline gallium arsenide (GaAs). Characteristics of MBE: Following are some of the important characteristics of MBE, 1. Low growth rate of ~ 1 monolayer (lattice plane) per sec. 2. Precise control of surface composition and morphology. 3. Grow films with good crystal structure. 4. A very smooth surface and interface is achievable through controlling the growth at the monoatomic layer level. 5. Low growth temperature (~ 550°C for GaAs). A low growth temperature limits diffusion and maintains hyper-abrupt interfaces, which are very important in fabricating two-dimensional nanostructures or multilayer structures such as quantum wells. 2.2. CHEMICAL METHODS 2.2.1. MICELLES AND MICROEMULSIONS Synthesis of nanoparticles in the cavities produced in microemulsion is a widely used method. Advantage of this method is the biocompatibility and biodegradability of synthesi zed materials. Biocompatibility is useful in novel applications like drug deliver y of nanomaterials and biodegradability is sometimes environmentally useful. Whenever two immiscible liquids are mechanically agitated or stirred together, they are known to form what is called "emulsion". The tendency of the liquids is such that the liquid in smaller quantity tries to form small droplets, coagulated droplets or layers so that they are all separated from the rest of the liquid (for example droplets of fat in milk). The droplet sizes in emulsions are usually larger than 100 nm upto even few millimetres. Emulsions are usually turbid in appearance. On the other hand, there is another class of immiscible liquids, known as microemulsions which are transparent and the droplets are in the range of ~ 1 to 100 nm. This is the size needed for the synthesis of nanornaterials. Microemulsions are stabilized using surfactants (surface stabilized active agents). When an organic liquid or oil (O), water (W) and surfactant (T) are mixed together, under some critical concentration, "micelles" or "inverse micelles" are formed, depending upon the concentrations of water and organic liquid. As shown in Fig. 19, micelles are formed with excess water and inverse micelles are formed in excess of organic liquid or oil. 17 Nanotechnology Unit 2: Preparation of nanomaterials Fig. 19: Formation of micelles and inverse micelles. As shown in Fig. 19, micelles are formed with the head groups floating in water, whereas tails and tail group filling the cavity along with organic liquid inside. Reverse is the case for inverse micelles. They can form various shapes as illustrated in Fig. 20. Fig. 20: Different types of micelles. The ratio of water (W), oil (O) and surfactant (T) is important to decide which type of micelle will be formed and can be represented in a ternary phase diagram using a triangle (Fig. 21). Fig. 21: Ternary phase diagram of water (W), oil (O) and surfactant (T) mixture. Composition can be determined by drawing lines parallel to all three sides of the triangle as shown in Fig. 21. Here point P denotes 50% water, 25% oil and 25% surfactant. A modified phase diagram known as "Winsor Diagram" also can be constructed for finer details (not shown here). The critical micelle concentration (CMC) depends upon all W, O and T concentrations as is evident from above diagram. As shown in Fig. 22, the effect of T is to reduce the surface tension of water dramatically below CMC and remain constant above it, as the organic solvent concentration 18 Nanotechnology Unit 2: Preparation of nanomaterials is kept on increasing. Organic solutes also reduce the surface tension to some small extent. If there are any electrolytes used, they slightly increase the surface tension. Fig. 22: Surface tension vs temperature. There are four types of surfactants in general: 1. Cationic − for example CTAB, C16H33N(CH3)3+Br− 2. Anionic − e.g. sulfonated compounds with general formula R-SO3−Na+ where R is CnH2n+1 3. Nonionic − for example R- (CH2-CH2-O)20-H 4. Amphoteric − some properties are similar to ionic and some to nonionic surfactants as in betaines. A large number of narioparticles of (metals, semiconductors and insulators) cobalt, copper, CaCO3, BaSO4, CdS, ZnS etc. have been synthesized using microemulsions or inverse micelles. As an example, consider the synthesis of cobalt nanoparticles. A reverse miceller solution of water and oil can be stabilized us ing a monolayer of surfactant like sodium bis (2-ethylhexyl) sulfosuccinate or Na(AOT). The droplet diametre is controlled simply by controlling the amount of water. Two miceller solutions having same diameter of droplets can be formed. Thus one solution should have Co(AOT) 2, i.e. cobalt bis (2-ethylhexyl) sulfosuccin ate and the other should have sodium tetrahydroborate (NaBH4 i.e. sodium borohydride). When two solutions are mixed together the solution appears clear but the colour changes from pink to black. One can find by electron microscopy or some other analysis that cobalt nanoparticles are formed. NOTE 1: AMPHIPHILIC MOLECULES IN LIQUIDS If amphiphilic molecules are spread in an aqueous solution, they try to stay at air-solution interface with hydrophobic groups in air and hydrophilic groups in the solution (see Fig. 23 (a)). Such molecules are known as surfactants (surface active agents). Fig. 23: Amphiphilic molecules in aqueous solutions 19 Nanotechnology Unit 2: Preparation of nanomaterials Consider now a situation in which a hydrocarbon molecule solution is put in an aqueous medium. As shown in Fig. 23 (b), the hydrocarbon solution itself would be separated from aqueous solution and float on it. When surfactant molecules are mixed in large quantity in aqueous solution, then they would try to form what are known as 'micelles' and 'inverse-micelles' when aqueous solution is mixed in oil. In micelles, the head groups float in water and tails are inside, whereas tails point outwards in case of inverse micelles.' NOTE 2: SURFACE TENSION OF LIQUIDS Surface tension of a liquid can change if some electrolyte, organic or surfactant solutes are added. General behaviour is shown in Fig. 24. a) b) Fig. 24: For surfactant molecules γ decreases rapidly upto certain concentration known as critical miceller concentration (c.m.c.). 2.2.2. CHEMICAL VAPOUR DEPOSITION (CVD) Chemical vapour deposition (a hybrid method using chemicals in vapour phase) is conventionally used to obtain coatings of a variety of materials viz. inorganic or organic materials. It is widely used in industry because of its relatively simple instrumentation, ease of processing, economical viability and possibility of depositing different types of materials. Under certain deposition conditions nanocrystalline films or single crystalline films can also be grown. Basically CVD process can be considered as a transport of reactant vapour or reactant gas towards the substrate (Fig. 19) kept at some high temperature where the reactant cracks into different products which diffuse on the surface (undergo some chemical reaction at appropriate site), nucleate and grow to form the desired material film. The by-products created on the substrate have to be transported back to the gaseous phase removing them from the substrate. Fig. 19: Basic concepts of chemical vapour deposition (CVD) process and CVD chamber. 20 Nanotechnology Unit 2: Preparation of nanomaterials Gas or Vapours of desired material may be often pumped into reaction chamber using some carrier gas (Fig. 19). In some cases the reactions may occur through aerosol formation in gas phase. There are various processes such as reduction of gas, chemical reaction between different source gases, oxidation or some disproportionate reaction by which CVD can proceed. However, it is preferable that the reaction occurs at the substrate rather than in the gas phase. Usually temperature ~ 300 to l200°C is used at the substrate and gas pressures in the range of 0.1 torr to l.0 torr are used. Growth rate and film quality depend upon the gas pressure and the substrate temperature. When the growth takes place at low temperature, it is limited by the kinetics of surface reaction. At intermediate temperature, it is limited by mass transport, i.e. supply of reacting gases to the substrate. Here the reaction is faster and supply of reactants is slower. At high temperature, growth rate reduces due to desorption of precursors from the substrate. GROWTH MECHANISMS: When two types of atoms or molecules, say P and Q, are involved in the desired thin film formation, then there are two mechanisms by which growth can take place. A. Langmuir-Hinshelwood mechanism: Both P and Q type of atoms/molecules are adsorbed on the substrate surface and interact there to produce PQ. When one species is adsorbed in excess of the other, the growth depends on the availability of adsorption sites for both P and Q as shown in a schematic diagram Fig. 20. Fig. 20: Langmuir-Hinshelwood mechanism of growth. B. Elay-Riedel mechanism: One species say Q adsorbs on the substrate and the species P from gas phase interacts with Q. Thus, there is no sharing of sites. This type of mechanism is known as Elay-Riedel mechanism and is as shown in Fig. 21. Fig. 21: Elay-Riedel mechanism of Growth 21 Nanotechnology Unit 2: Preparation of nanomaterials TYPES OF CVD: A variety of CVD methods and CVD reactors have been developed, depending on the types of precursors used, the deposition conditions applied and the forms of energy introduced to the system to activate the chemical reactions desired for the deposition of solid films on substrates. For example, when metal organic compounds are used as precursors, the process is generally referred to as metal organic CVD (MOCVD), and when plasma is used to promote chemical reactions, this is a plasma enhanced CVD (PECVD). There are many other modified CVD methods, such as low pressure CVD (LPCVD), laser enhanced or assisted CVD, and aerosolassisted CVD (AACVD). 2.2.3. SOL-GEL PROCESS As the name suggests sol-gel involves two types of materials or components, 'sol' and 'gel'. Sols are solid particles in a liquid (see Fig. 22). They are thus a subclass of colloids. Gels are nothing but a continuous network of particles with pores filled with liquid (or polymers containing liquid). A sol-gel process involves formation of 'sols' in a liquid and then connecting the sol particles (or some subunits capable of forming a porous network) to form a network. By drying the liquid, it is possible to obtain powders, thin films or even monolithic solid. Fig. 22: (a) sol, (b) gel and monolithic solid (aerogel). Sol-gel method is particularly useful to synthesize glass materials, ceramics or metal oxides although sulphides, borides and nitrides also are possible. Nowadays the sol-gel process is quite often used for the fabrication of a variety of nanomaterials. Usually metal alkoxides and metal chlorides are used as precursors. Figure 23 shows the typical sol-gel process. Fig. 23: Schematic diagram of sol-gel process. 22 Nanotechnology Unit 2: Preparation of nanomaterials POLYMERISATION OF METAL ALKOXIDE: Metal alkoxides (R-O-M) are organic compounds with one or more metal atoms in the molecule. They are like alcohols (R-OH) with a metal atom M replacing the hydrogen H in the hydroxyl group. Metal alkoxides are widely used in sol-gel synthesis as ideal chemical precursors. Silicon tetraethoxide or tetraethyl orthosilicate (TEOS) is an example for metal alkoxide. Its chemical formula is Si(OC2H5)4 or Si(OR)4 where the alkyl group R = C2H5. Hydrolysis and condensation are the two essential processes which takes place in sol-gel process. Hydrolysis: Metal alkoxides (R-O-M) react readily with water, and a hydroxyl ion becomes attached to the metal atom during the reaction. This reaction is called hydrolysis. M-O-R + H2O → M-OH + R-OH e.g.: Si(OR)4 + 4 H2O → Si(OH)4 + 4 R-OH During the completion of hydrolysis process, all of the OR groups are replaced by OH groups, depending on the amount of water and catalyst present. Any intermediate species [(OR)2-Si-(OH)2 or [(OR)3-Si(OH)] will be considered the result of partial hydrolysis. Condensation: Two partially hydrolyzed molecules can link together in a condensation reaction to form a siloxane [Si-O-Si] bond: Water condensation: M-OH + HO-M → M-O-M + H2O e.g.: (OR)3-Si-OH + HO-Si-(OR)3 → [(OR)3Si-O-Si(OR)3] + H-O-H Alcohol condensation: M-O-R + HO-M → M-O-M + R-OH e.g.: (OR)3-Si-OR + HO-Si-(OR)3 → [(OR)3Si-O-Si(OR)3] + R-OH Thus, polymerization of metal alkoxides is associated with the formation of a 1, 2, or 3dimensional network of siloxane. [Si-O-Si] bonds along with the production of H-O-H and R-OH species. This will give the required sol. Then, the sol is aged for a certain time to get long chains of siloxane bonds, removed by hydroxyl groups, resulting in thickening of the sol to form the gel. Finally, this gel is heated up in atmospheric air to get SiO 2. The condensation liberates a small molecule, such as water or alcohol. This type of reaction can continue to build larger and larger silicon-containing molecules by the process of polymerization. 2.3. LITHOGRAPHY Mainly, there are two approaches for the fabrication of nanomaterials, bottom-up and topdown approaches. In the earlier sections of this chapter, we have seen few methods to make nanoparticles and thin films (or multilayers) [e.g. sputtering, CVD, thermal evaporation, pulsed laser deposition, molecular beam epitaxy etc]. These methods are popularly known as bottom-up approach. In bottom-up approach, atoms and molecules are assembled so as to form nanomaterials of required size and shape by controlled deposition or reaction parameters. In top-down approach, usually a bulk material is taken and machined to modify into the desired size, shape and product. Ball milling is an example of top-down approach. Lithography may be considered as a hybrid approach because growth is bottom-up while etching is top-down. But nanolithography is commonly a bottom-up approach. Both approaches play a very important role in nanotechnology. There are advantages and disadvantages in both 23 Nanotechnology Unit 2: Preparation of nanomaterials approaches. The imperfection of the surface structure is the drawback of top-down approach. For instance, in lithographic techniques the processed patterns contain crystallographic damages in addition to the defects which occur during the etching process. Such imperfections would have a significant impact on physical and chemical properties of nanomaterials, since their surface to volume ratio is very large. The word lithography has originated from Greek words "litho" (which means stone) and "gramma" (which means writing). Therefore, lithography literally means carving a stone or writing on a stone. Now, it is used to mean a process in which a sample is patterned by removing some part of it (i.e. etching) or sometimes even organizing some material on a suitable substrate. Lithography is very extensively used in electronics industry to obtain integrated circuits (IC) or very large scale integration (VLSI) on small piece of semiconductor substrate often called a "chip". Over the last 3-4 decades, different lithography techniques like photolithography, X-ray lithography, electron beam lithography and some others have been developed. They depend upon using photons or particle radiations for carving the materials. The lithography technique involves transfer of some pre-designed geometrical pattern (called master or mask) on a semiconductor (like silicon) or directly patterning (often known as writing) using suitable radiation. Mask is usually prepared by creating radiation opaque and transparent regions on glass or some other material. Predesigned patterns can be transferred on a substrate much faster as compared to direct writing. Direct writing being a slower process is overall expensive. PRINCIPLE AND PRODUCE OF LITHOGRAPHY: Common principle in most of the lithography techniques is to expose a material sensitive to either electromagnetic radiation or to particles at some regions. Such a radiation sensitive material is known as resist. The selection of area is made using a mask which is transparent in some regions and opaque in the other regions. This causes selective exposure of the resist, making it weaker or stronger compared to unexposed material depending upon the type of the resist being used. By removing the exposed or unexposed material in suitable chemicals or plasma, desired pattern is obtained. This may be done in a number of steps depending upon the pattern and materials involved. Fig. 24 depicts schematically various steps involved in photolithography to transfer a pattern on the surface of some semiconductor. A thin film coating of a metal (e.g. chromium) is deposited on a suitable substrate (e.g. glass or silicon). A positive or a negative photo-resist (usually some polymer) is coated on metal thin film. Positive photo-resist material has the property that, when exposed to the appropriate radiation it degrades or some chemical bonds are broken. Negative resist on the other hand is a material, which hardens (cross-links) on exposure to a radiation. A mask is placed between the resist coated substrate and the source of light. By using a suitable chemical (developer), the weakened portion is removed (or image is developed). Remaining unexposed part also can be removed by appropriate chemical treatment. The remaining material can be dissolved in one step and the hardened material in another step. Depending on the radiation used like visible light, X-rays, electrons, ions etc. the lithography name is tagged with it. 24 Nanotechnology Unit 2: Preparation of nanomaterials Fig. 24: Photolithography process steps: (1) surface is coated with metal, (2) coating of photoresist on the substrate, (3) mask placed over upper layer, (4) exposed to UV radiation, (5) resist development and striping and (6) etching to get final pattern. 2.3.1. PHOTOLITHOGRAPHY It is possible to use visible, ultraviolet, extreme ultraviolet (EUV) or X-rays to perform lithography and wherever possible lasers are also used. Highest resolution of the generated features ultimately depends upon the wavelength of radiation used and interaction of radiation with matter as well as mask and optical elements used. Smaller the wavelength used smaller can be the feature size which is limited by diffraction limit, ~ λ/2. Depth of focus depends upon the penetration of incident radiation. For the lithography using electromagnetic radiation, optical elements and masks have to be used for various purposes. In the visible range (~700 nm to 400 nm) glass lenses and masks can be used. In the UV range fused silica or calcium fluoride lenses are used. There are three methods (see Fig. 25) viz. "proximity", "contact" and "projection" which can be used to pattern a substrate. Fig. 25: (1) mask is close to the photo-resist, (2) mask is in contact with the resist and (3) focused beam is scanned through the mask. As the name suggests, in 'proximity' method, mask is held close to the photo-resist coated metalized substrate, whereas in 'contact' method the mask is in contact with photo-resist. In both proximity and contact methods a parallel beam of light fal1s on the mask, which transmits the radiation through some windows but blocks through opaque parts. Although better resolution is 25 Nanotechnology Unit 2: Preparation of nanomaterials achieved with contact method as compared to proximity method, in contact method the mask gets damaged faster. In case of 'projection' method a focused beam is scanned through the mask, which allows good resolution to be achieved along with the reduced damage of the mask. However, scanning is a slow process and also requires scanning mechanism adding to the cost. LITHOGRAPHY USING UV LIGHT AND LASER BEAMS Using monochromatic light in the visible to UV range features as small as 1 to 1.5 µm size can be routinely obtained. Often g-line [i.e. green colour] (436 nm) from mercury lamp is used. Laser beam of KrF (248 nm) or ArF (193 nm) also are employed reaching ~150 nm as the smallest feature size. However to obtain feature size below ~ 100 nm using photons is a difficult task. LITHOGRAPHY USING X-RAY Smaller features are possible to obtain by employing X-rays also. However, it is difficult to make suitable masks for X-ray lithography. X-rays in the 0.1 to 5 nm range are used with appropriate metal masks in proximate geometry. Absorption of X-rays in materials not only depends upon the thickness of the material but is also complicated by the presence of absorption edges. Depending upon the wavelength of X-rays used, metals of suitable elements are chosen. Metal masks are fabricated in such a way that through thin portions rays are transmitted and absorbed in thicker regions. Gold masks are often used. The masks themselves are made using electron beam lithography discussed in the next section. 2.3.2. PARTICLE BEAM LITHOGRAPHY We know that, all the moving particles have associated wavelength λ known as de Broglie wavelength given by, h λ= mv Where h is Planck's constant, m is the mass and v is the velocity of the particle. All kinds of particles can in principle be used. But to achieve high resolution λ should be as small as possible. Thus, large mass and large velocity of particle makes it possible to get adequate resolution. In fact it is possible using neutral atoms, ions or electrons to bring down the particle associated wavelength to any desired value, even as small as 0.1 nm. However, ultimate resolution depends upon the interaction of incident particles with resist material. Under certain conditions, features as small as 2 nm have been patterned. Due to various reasons like electrons can be easily generated, accelerated and focused, electrons are preferred for lithography purpose and often used. ELECTRON BEAM LITHOGRAPHY Figure 26 shows schematically electron beam lithography set up. It is very similar to a scanning electron microscope (SEM) and requires vacuum (~ 10 −5 torr). Sometimes SEM is modified in order to use it as a lithography set up. Electron beam lithography is a direct writing method, i.e. no mask is required to generate a pattern. Rather, patterns or masters required for other lithography processes (like optical lithography and soft lithography) can be generated using electron beam lithography. Electrons with high energy (usually larger than ~ 5 keV) are incident on the photo-resist. Here also positive or negative photo-resists can be used. Common positive resists are poly-methyl26 Nanotechnology Unit 2: Preparation of nanomaterials methacrylate (PMMA) and polybutane-l-sulphone (PBS). Negative resist often used in electron beam lithography is poly-glycidyl-methacrylate co-ethyl-acrylate (COP). Developers used are methyl-isobutyl-ketone (MIBK) and isopropylalcohol (IPA) in 1:1 ratio. Fig. 26: Electron beam lithography set up. A focused electron beam in electron beam lithography is used in two modes, viz. 'vector scan' or 'raster scan'. In vector scan, the electron beam 'writes' on some specified region. After one region is completed the X-Y scanning stage on which the substrate to be patterned is mounted moves. During its movement electron beam is put off. Then a new region is selected and ' written' with the beam. This is continued until whole pattern is generated. In 'raster scan' the beam is rastered or moved continuously over a small area, line by line. The X-Y stage of the sample moves at right angles to the beam. The beam is turned off or turned on depending upon the pattern. Although very high resolution (~50 nm) is routinely possible using this lithography, due to scanning mode it is rather slow. For example, if the optical lithography can generate 40 patterns with 1µm resolution in one hour, only five similar patterns would be generated with electron beam. However, larger layer depth is an added attraction of electron beam lithography as compared to optical beam lithography. ION BEAM LITHOGRAPHY Very small features (~5-10 nm) can be written using high-energy ion beams. Major advantages of using ion beams is that resists are more sensitive to ions as compared to electrons and have low scattering in the resist as well as from the substrate. Commonly used ions are He+, Ga+ etc. with energy in the 100-300 keV range. ********************************************************************************** 27 Nanotechnology Unit 2: Preparation of nanomaterials MODEL QUESTIONS 1. Explain bottom-up and top down approaches for fabricating of nanostructures with examples. What are the advantages and disadvantages of both methods? 2. What is ball mill? Explain common ball miller and planetary ball miller with neat figures. 3. Explain with neat schematic lay out: a) inert gas condensation, b) arc discharge method and c) laser ablation. 4. What is sputtering? Explain DC, RF and magnetron sputtering with neat diagrams. 5. What is pyrolysis? Explain the spray pyrolysis and flame pyrolysis with the neat diagrams. 6. Explain thermal/resistive evaporation with its schematic lay out? List the advantages and disadvantages thermal evaporation. 7. Explain pulsed laser deposition with its schematic layout? List its advantages. 8. What is meant by epitaxy? Explain molecular beam epitaxy with its schematic layout. List its characteristics. 9. What are Microemulsions? Explain the formation and types of micelles along with suitable diagrams. 10. Explain chemical vapour deposition process and growth mechanism with neat diagrams. 11. Explain sol-gel process with schematic diagram and also polymerization of metal alkoxides. 12. What is lithography? Explain the basic principle and procedure involved in lithography with neat diagram. 13. Explain photo-lithography with neat figure. Mention the types of photolithography. 14. Explain electron beam lithography with neat figure. ********************************** END ************************************** 28