Thermodynamics Analysis of Engine Cycles
advertisement

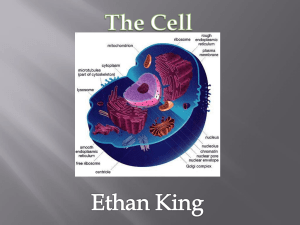
Thermodynamics Analysis of Engine Cycles Summary Thermodynamics is the science of the relationship between heat, work, and systems that analyze energy processes. The energy processes that convert heat energy from available sources such as chemical fuels into mechanical work are the major concern of this science. Thermodynamics consists of a number of analytical and theoretical methods which may be applied to machines for energy conversion. First Law of Thermodynamics The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes: Change in internal Heat added to - Work done by energy = the system the system The first law makes use of the key concepts of internal energy, heat, and system work. It is used extensively in the discussion of heat engines. It is typical for chemistry texts to write the first law as ΔU=Q+W. It is the same law, of course - the thermodynamic expression of the conservation of energy principle. It is just that W is defined as the work done on the system instead of work done by the system. In the context of physics, the common scenario is one of adding heat to a volume of gas and using the expansion of that gas to do work, as in the pushing down of a piston in an internal combustion engine. In the context of chemical reactions and process, it may be more common to deal with situations where work is done on the system rather than by it. Enthalpy Four quantities called "thermodynamic potentials" are useful in the chemical thermodynamics of reactions and non-cyclic processes. They are internal energy, the enthalpy, the Helmholtz free energy and the Gibbs free energy. Enthalpy is defined by H = U + PV where P and V are the pressure and volume, and U is internal energy. Enthalpy is then a precisely measurable state variable, since it is defined in terms of three other precisely definable state variables. It is somewhat parallel to the first law of thermodynamics for a constant pressure system Q = ΔU + PΔV since in this case Q=ΔH It is a useful quantity for tracking chemical reactions. If as a result of an exothermic reaction some energy is released to a system, it has to show up in some measurable form in terms of the state variables. An increase in the enthalpy H = U + PV might be associated with an increase in internal energy which could be measured by calorimeter, or with work done by the system, or a combination of the two. The internal energy U might be thought of as the energy required to create a system in the absence of changes in temperature or volume. But if the process changes the volume, as in a chemical reaction which produces a gaseous product, then work must be done to produce the change in volume. For a constant pressure process the work you must do to produce a volume change ΔV is PΔV. Then the term PV can be interpreted as the work you must do to "create room" for the system if you presume it started at zero volume. System Work When work is done by a thermodynamic system, it is usually a gas that is doing the work. The work done by a gas at constant pressure is: The line from a to b represents an expansion of a gas at constant pressure. The work done is the area under the curve. For non-constant pressure, the work can be visualized as the area under the pressure-volume curve which represents the process taking place. The more general expression for work done is: The integral gives the exact area under the curve which is equal to the work. Work done by a system decreases the internal energy of the system, as indicated in the First Law of Thermodynamics. System work is a major focus in the discussion of heat engines. Second Law of Thermodynamics The second law of thermodynamics is a general principle which places constraints upon the direction of heat transfer and the attainable efficiencies of heat engines. In so doing, it goes beyond the limitations imposed by the first law of thermodynamics. The maximum efficiency which can be achieved is the Carnot efficiency. Second Law: Heat Engines Second Law of Thermodynamics: It is impossible to extract an amount of heat QH from a hot reservoir and use it all to do work W. Some amount of heat QC must be exhausted to a cold reservoir. This precludes a perfect heat engine. This is sometimes called the "first form" of the second law, and is referred to as the Kelvin-Planck statement of the second law. Second Law: Entropy Second Law of Thermodynamics: In any cyclic process the entropy will either increase or remain the same: Entropy: a state variable whose change is defined for a reversible process at T where Q is the heat absorbed. Entropy: a measure of the amount of energy which is unavailable to do work. Entropy: a measure of the disorder of a system. Entropy: a measure of the multiplicity of a system. Since entropy gives information about the evolution of an isolated system with time, it is said to give us the direction of "time's arrow" . If snapshots of a system at two different times shows one state which is more disordered, then it could be implied that this state came later in time. For an isolated system, the natural course of events takes the system to a more disordered (higher entropy) state. Heat Engine Processes Heat engine processes are shown on a PV diagram. Besides constant pressure, volume and temperature processes, a useful process is the adiabatic process where no heat enters or leaves the system. Heat Engine Processes http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/heatengcon.html#c1 The Diesel Engine The diesel internal combustion engine differs from the gasoline powered Otto cycle by using a higher compression of the fuel to ignite the fuel rather than using a spark plug ("compression ignition" rather than "spark ignition"). In the diesel engine, air is compressed adiabatically with a compression ratio typically between 15 and 20. This compression raises the temperature to the ignition temperature of the fuel mixture which is formed by injecting fuel once the air is compressed. The ideal air-standard cycle is modeled as a reversible adiabatic compression followed by a constant pressure combustion process, then an adiabatic expansion as a power stroke and an iso-volumetric exhaust. A new air charge is taken in at the end of the exhaust, as indicated by the processes a-e-a on the diagram. Since the compression and power strokes of this idealized cycle are adiabatic, the efficiency can be calculated from the constant pressure and constant volume processes. The input and output energies and the efficiency can be calculated from the temperatures and specific heats: It is convenient to express this efficiency in terms of the compression ratio rv = V1/V2 and the expansion ratio rE = V1/V3. The efficiency can be written Now using the ideal gas law PV= n R T and g= CP/CV, this can be written Now using the fact that Va = Vd = V1 and Pc=Pb from the diagram Dividing the numerator and denominator by V1Pc Now making use of the adiabatic condition PVg= constant, the efficiency can be written The Otto Engine cv – Specific Heat constant volume γ – Specific Heat Ratio p – pressure T – temperature V – volume f – fuel/air ratio Q – Fuel heating value Cps –cycles per secomd P – power Compression stroke: Work per cycle: Engine Power: Combustion: Power Stroke: Ideal Otto Cycle On the figure we show a plot of pressure versus gas volume throughout one cycle. We have broken the cycle into six numbered stages based on the mechanical operation of the engine. For the ideal four stroke engine, the intake stroke (1-2) and exhaust stroke (6-1) are done at constant pressure and do not contribute to the generation of power by the engine. During the compression stroke (2-3), work is done on the gas by the piston. If we assume that no heat enters the gas during the compression, we know the relations between the change in volume and the change in pressure and temperature from our solutions of the entropy equation for a gas. We call the ratio of the volume at the beginning of compression to the volume at the end of compression the compression ratio, r. Then where p is the pressure, T is the temperature, and γ is the ratio of specific heats. During the combustion process (3-4), the volume is held constant and heat is released. The change in temperature is given by where Q is the heat released per pound of fuel which depends on the fuel, f is the fuel/air ratio for combustion which depends on several factors associated with the design and temperature in the combustion chamber, and Cv is the specific heat at constant volume. From the equation of state, we know that: During the power stroke (4-5), work is done by the gas on the piston. The expansion ratio is the reciprocal of the compression ratio and we can use the same relations used during the compression stroke: Between stage 5 and stage 6, residual heat is transferred to the surroundings so that the temperature and pressure return to the initial conditions of stage 1 (or 2). During the cycle, work is done on the gas by the piston between stages 2 and 3. Work is done by the gas on the piston between stages 4 and 5. The difference between the work done by the gas and the work done on the gas is shown in yellow and is the work produced by the cycle. We can calculate the work by determining the area enclosed by the cycle on the p-V diagram. But since the processes 2-3 and 4-5 are curves, this is a difficult calculation. We can also evaluate the work W by the difference of the heat into the gas minus the heat rejected by the gas. Knowing the temperatures, this is an easier calculation. The work times the rate of the cycle (cycles per second cps) is equal to the power P produced by the engine. The efficiency is: Combustion of fossil fuel, Using Thermo Utilities, MS Excel Add-ins A fossil fuel with the following composition by mass: C 70%; H 18.5%; O 3%; N 4%; S 1.5%; ash 3% has been burned in a boiler, when 100% excess air is supplied. Combustion efficiency is 0.75 Calculate: 1. the stoichiometric air-to-fuel (A/F) ratio 2. the A/F ratio 3. analysis of combustion products (dry and wet) 4. temperature of exhaust gases Air is supplied at atmospheric pressure and 18 C with 0.008 specific humidity. The fuel has an average temperature of 35 C when enters the boiler. Use Dulong formula to estimate the net calorific value of the fuel. The specific heat capacity of fuel is 3.2 kJ/kg,K. Combustion Equations Combustion equation for coal: C + O2 => CO2 (12 kg C)+(32 kg O) => (34 kg CO2) Combustion equation for hydrogen: 2 H2 + O2 => 2 H2O (4 kg H)+(32 kg O) => (36 kg H2O) Combustion equation for sulphur: S + O2 => SO2 (32 kg S) + (32 kg O) => (64 kg SO2) Fuel Analysis . Constituent Mass fraction . . . Required oxygen Product mass . kg/kg fuel kg/kg fuel Carbon 0,700 1,867 2,567 Hydrogen 0,185 1,480 1,665 Oxygen 0,030 -0,020 0,010 Nitrogen 0,040 0,000 0,040 Sulphur 0,015 0,015 0,030 Ash 0,030 0,000 0,030 . 1,000 3,342 4,342 Analysis of Supplied Air . . . Specific Humidity 0,008 . . Composition by mass . . . Constituent Dry Air Humid Air . N2 0,76280 0,75670 . O2 0,23290 0,23104 . CO2 0,00300 0,00298 . Ar 0,00130 0,00129 . H2O 0,00000 0,00800 . SO2 0,00000 0,00000 . . 1,00000 1,00000 . Air required per kg of fuel 14,46 Stoichiometric kg/kg A/F ratio Excess Air 1 . . Actual A/F ratio kg/kg 28,92757 . . Exhaust Gases . Wet Mass Dry Mass Constituent Mass Composition Composition N2 21,92942 0,73373 0,78344 O2 3,34167 0,11181 0,11938 CO2 2,65276 0,08876 0,09477 Ar 0,03730 0,00125 0,00133 H2O 1,89642 0,06345 0,00000 SO2 0,03000 0,00100 0,00107 . 29,88757 1,00000 1,00000 Exhaust Gases . . Volume Constituent Kg/kmol Mole Fraction Composition N2 28 0,02620 0,74260 O2 32 0,00349 0,09901 CO2 44 0,00202 0,05716 Ar 40 0,00003 0,00088 H2O 18 0,00353 0,09990 SO2 64 0,00002 0,00044 0,03529 1,00000 . . Mass balance . . . Fuel 1,00000 . . Supplied Air 28,92757 . . . 29,92757 . . . . . . Exhaust Gases 29,88757 . . Ash 0,03000 . . . 29,91757 . . Dulong suggests the following formula for gross calorific value (GCV) of fossil fuels when oxygen content is less than 10% GCV = 337 C +1442 (H - O/8) + 93 S GCV is in (kJ/kg). C, H, O, S are percentages on weight basis for carbon, hydrogen, oxygen and sulphur. The net calorific value for a constant pressure combustion is: NCV = GCV - mc * hfg mc is the mass of condensate per unit quantity of fuel and hfg is the latent heat of steam at 25 degree Celsius which is 2442 kJ/kg. Supplied Air Temp. 18 . . Fuel Cp 3,2 kJ/(kg.K) . Gross Calorific Value, GCV 49866 kJ/kg . Net Calorific Value, NCV 49711 kJ/kg . Combustion efficiency 0,75 . . . . . . . Enthalpy Mass Flow m*h . kJ/kg kg/s kJ/s Supplied Air 38,31 28,93 1108,10 Fuel 64,00 1,00 64,00 Fuel Energy Supplied 49710,80 . . 1,00 37283,10 . 38455,21 Exhaust Gases 1284,94 29,93 38455,21 Exhaust Gases Temp 964, C . Topic group Topic Summary Gasoline fuel system principles Gasoline fuel The properties of gasoline must be balanced to give satisfactory engine performance over a wide range of operating conditions including heat, altitude, and driving patterns. The more effectively liquid gasoline is changed into vapor, the more efficiently it burns in the engine. Gasoline fuel characteristics There are several important characteristics of hydrocarbon based fuels: volatility, octane rating & energy content. The most important characteristic of gasoline is its Research Octane Number (RON) or octane rating, which is a measure of how resistant gasoline is to premature detonation (knocking). A range of fuel additives are used to change a fuel's performance characteristics. Controlling fuel burn Detonation is a violent collision of flame fronts in the cylinder, caused by uncontrolled combustion. The sudden rise in pressure can cause a knocking sound. Stoichiometric ratio Stoichiometric ratio is the air-fuel ratio necessary for complete combustion. Air density The density of air is its mass per unit volume. Fuel supply system EFI is a circulation system. A pump draws fuel from the tank and sends it to solenoid-operated injection valves, where pressure is maintained by a fuel pressure regulator. Excess fuel flows back to the tank through a return line. Pressure & vacuum As air pressure is reduced, a vehicle has to reduce the amount of fuel delivered to the engine to maintain the correct air-fuel ratio. Carburetion A light vehicle under normal conditions needs an airfuel ratio, by mass, of about 15 to 1. By volume, that's 11000 to 1. Carburetor system components The main components in most carbureted systems are a float chamber, a venturi, the throttle, idle circuit, main circuit, a choke and an accelerator pump. Carburetor systems Low speed and idling ports allow the engine to operate with a low throttle opening before the main Carburetor operation system is operating fully. Carbureted system components Metering jets The main jet size is selected to provide the best mixture for fuel economy. An extra jet supplies additional fuel for maximum power. Accelerating For acceleration, suddenly depressing the accelerator delivers extra fuel into the airstream. Carburetor barrels A two-stage carburetor has a primary throttle open only from idle to medium speeds. At higher speeds, the secondary throttle opens to admit more air-fuel mixture. The carburetor The carburetor atomises the fuel and mixes it with air, and controls the delivery of the correct mixture to the engine. Mechanical fuel pumps The mechanical fuel pump has a diaphragm separating two chambers. Moving the diaphragm down draws fuel into the pumping chamber. A spring then moves the diaphragm up, forcing fuel from the pump, into the carburetor. Electric fuel pumps An electric fuel pump operates with the ignition switched on. It can be controlled so that it operates only if the engine is running. Tanks & lines Most fuel tanks are in two parts joined by a weld around the flanges where the parts fit together. Baffles make the tank more rigid, prevent surging of fuel, and ensure fuel is available at the pickup tube. Fuel lines The fuel tank is connected to the engine by fuel lines. A return line may carry excess fuel back to the tank, to keep fuel system components cool. Charcoal canister Charcoal canisters are used in some emission systems as a means of preventing pollution to the atmosphere. Carburetor filters Carburetor filters are used to prevent particles from entering the fuel carburetion/injection components. EFI fuel supply system - EFI principles principles Basic EFI principles EFI systems employ injectors to spray the fuel into the intake system. An electronic control unit processes data received from various sensors to optimize the air fuel mixture at any given moment by adjusting the amount of fuel injected. EFI is a pressurised, indirect-injection system with solenoid-operated injectors. In multi-point injection, one injector is in each intake manifold runner. Single-point injection uses one or two injectors in a carburetor-like throttle-body. Air supply The design of the intake system determines how much air can be drawn into a cylinder at any given engine RPM. EFI can achieve uniform distribution of the air delivered to the cylinders. Air volume The amount of air entering the engine must be measured, so that the amount of fuel injected into it forms a mixture to suit the engine operating conditions at that time. Multi-point injection systems For any injection duration, if fuel is held at constant pressure, then, as manifold pressure varies, so does the amount of fuel delivered. That means fuel pressure must be held constant above manifold pressure. Simultaneous injection In multi-point injection, the injectors can all be triggered simultaneously, twice per cycle. In a throttle-body system, the central injector is normally triggered on each ignition pulse. With two injectors, alternate triggering may be used. Efficient combustion Adaptive learning is a form of feedback that lets fuel settings change as components age. The ECU memorizes its fuel settings for different operating conditions, and stores them for future use. EFI fuel supply system - Fuel pumps components EFI fuel pumps operate electrically to provide fuel under pressure to the fuel rail and the injectors. Fuel filters EFI fuel filters remove contaminants from the fuel, so that clean fuel can be supplied to the injectors. Tanks & lines Most fuel tanks are in two parts joined by a weld around the flanges where the parts fit together. Baffles make the tank more rigid, prevent surging of fuel, and ensure fuel is available at the pickup tube. Fuel lines The fuel tank is connected to the engine by fuel lines. A return line may carry excess fuel back to the tank, to keep fuel system components cool. Fuel rail The fuel rail supplies fuel to the injectors under constant pressure. Fuel pressure regulator The fuel pressure regulator controls the return of fuel to the fuel tank, to maintain the pressure in the fuel rail at a constant value above intake manifold pressure. Injectors Injectors are solenoid-operated valves which deliver fuel in the form of an atomised spray, into the intake manifold, or the intake ports. Tachometric relay The tachometer indicates engine RPM. Thermotime switch The thermotime switch senses engine coolant temperature, to control the operation of the cold start injector, during cranking conditions. EFI sensors EFI sensors include: wide band oxygen sensors, twin oxygen sensors, knock sensors, oil deterioration sensor, exhaust gas recirculation sensors and switches. Potentiometer A potentiometer is a mechanically variable resistor. Auxiliary air valves Auxiliary air valves allow additional air to bypass the throttle plate during cold start, and warm-up conditions. Idle speed control devices Idle speed control devices allow the preset idling speed to be maintained automatically when additional loads are placed on the engine, during idling conditions. Fuel system procedure Inertia sensors Inertia sensors shut off the fuel pump in the event of an accident, to minimize the danger of fuel spillage from a leak in the system. Replacing a fuel filter There are two types of fuel filter: carbureted system filter and EFI system filter. It is important to follow the correct procedure for the type of vehicle you are servicing. The objective of this procedure is to show you how to remove and replace a fuel filter.