pubdoc_12_16979_1444
advertisement
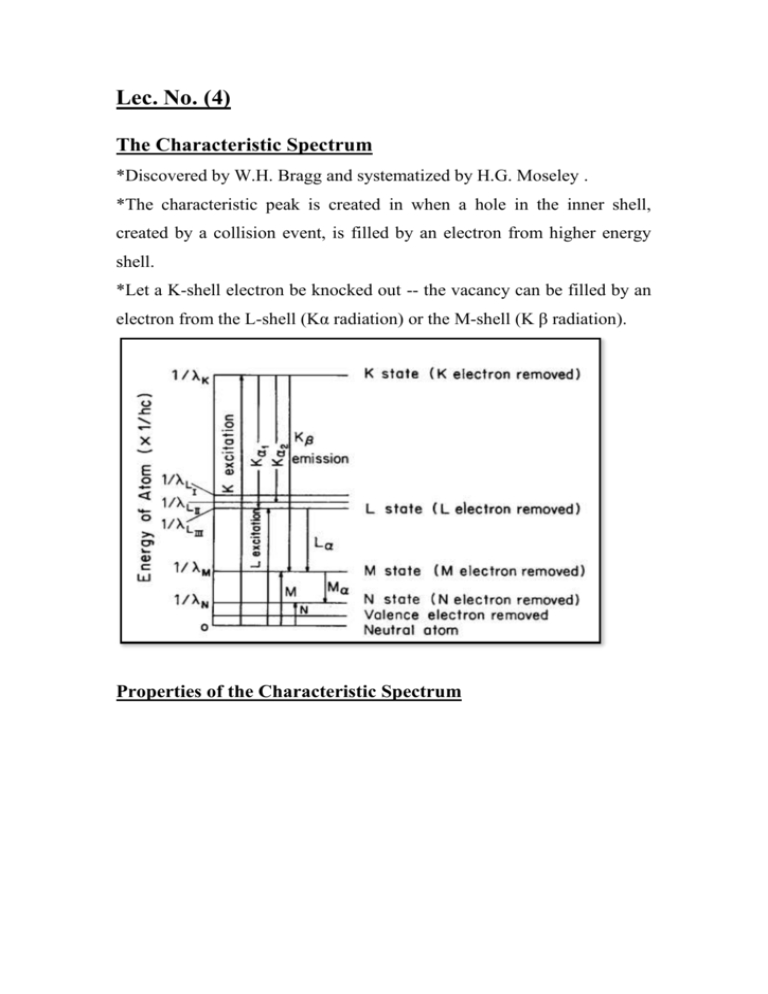
Lec. No. (4) The Characteristic Spectrum *Discovered by W.H. Bragg and systematized by H.G. Moseley . *The characteristic peak is created in when a hole in the inner shell, created by a collision event, is filled by an electron from higher energy shell. *Let a K-shell electron be knocked out -- the vacancy can be filled by an electron from the L-shell (Kα radiation) or the M-shell (K β radiation). Properties of the Characteristic Spectrum *The intensity of any characteristic line depends both on the tube current (i) and the amount by which the applied voltage V exceeds the critical excitation voltage for that line. For a K-line. *Characteristic lines are also very narrow, most of them less than 0.001 Å wide (Full Width At Half Maximum). *High intensity and narrow K-lines makes x-ray diffraction possible, since it generally requires the use of monochromatic radiation. Absorption coefficients of lead Properties of the Absorption Coefficient *There is a sharp discontinuity in the dependence of the absorption coefficient on energy (wavelength) at the energy corresponding to the energy required to eject an inner-shell electron. *The discontinuity is known as an absorption edge. *Away from an absorption edge, each “branch” of the absorption curve is given by: μ /ρ=k λ3 Z3 k : a constant. Z :atomic number of absorber. Lec. No. (6) Question (1) X-rays are generated by making the electrically charged particles (electrons) with sufficient kinetic energy in vacuum collide with cathode, as widely used in the experiment of an X-ray tube. The wavelength distribution and intensity of continuous X-rays are usually depending upon the applied voltage. A clear limit is recognized on the short wavelength side. (1) ) Find the relation of the shortest wavelength limit (SWL) of X-rays generated with the applied voltage V , when an electron loses all energy in a single collision. (2) Estimate the speed of electron before collision when applied voltage is 30,000V and compare it with the speed of light in vacuum.(H.W) Answer 1)λSWL = c/νmax = c e V/h = 1.602 * 10_19 * 2.998 * 108 * V /6.626 * 10_34 =0.7248 *1023 V 2) EK=eV=1/2 mg2 Question (2) Kα1 radiation of Fe is the characteristic X-rays emitted when one of the electrons in L shell falls into the vacancy produced by knocking an electron out of the K-shell, and its wavelength is 0.1936 nm. Find the energy difference related to this process for X-ray emission? Sol. Consider the process in which an L shell electron moves to the vacancy created in the K shell of the target (Fe) by collision with highly accelerated electrons from a filament. The wavelength of the photon released in this process is given by λ , (with frequency ν). We also use Planck’s constant h of (6.626 *10_34 Js) . Energy per photon is given by E= hν= hc/λ Using Avogadro’s number NA( 0.6022 * 1024), one can obtain the energy difference ΔE related to the X-ray release process per mole of Fe. ΔE = NA h c / λ ΔE = 6.1979 *108 J/mole Question (3) There is a substance of linear absorption coefficient. (1) Obtain a simple relation to give the sample thickness x required to reduce the amount of transmitted X-ray intensity by half. (2) Calculate also the corresponding thickness of Fe-17 mass % Cr alloy .density( D= 7.76 * 106 g/m3 ) for Mo-K˛ radiation. x = 0.693/µ The values of mass absorption coefficients of Fe and Cr for the Mo-K˛ radiation are 37.6 and 29.9 cm2/g obtained from Appendix, respectively. The concentration of Cr is given by 17 mass %, so that the weight ratio of two alloy components can be set as wFe = 0.83 and wCr = 0.17. Then, the mass absorption coefficient of the alloy is expressed in the following Next, note that the unit of the density of the Fe–Cr alloy is(7.76 g/cm3) (H.W) Calculate the mass absorption coefficient of lithium niobate (LiNbO3) for Cu-K˛ radiation. Lec. No. (7) Filters When the energy of a photon beam is just above the excitation potential or absorption edge of a material, that material strongly absorbs the given photon beam. If another substance can be found that has an absorption edge between the Kα and Kβ lines of the incident photon beam, this other substance can be used to significantly reduce the intensity of the Kβ line relative to the Kα line. The absorption edges of elements with Z Filter = ZTarget - 1 (or - 2) meet this requirement. The thickness of the filtering material is usually chosen to reduce the intensity of the Kβ line by a factor of 100 while reducing the intensity of the Kα line by a factor of 10 or less .the propose from filter to absorb the Kβ component much more strongly than Kα component ,because of the abrupt change in its absorption coefficient between two wavelengths . Lec. No. (8) Production of x-rays x-ray tube: any x-ray tube must contain : 1)a source of electrons . 2)high accelerating voltage. 3)metal target. The most of kinetic energy of the electrons is converted into heat in the target ,and must be cooled by water to prevent its melting. x-ray tubes can be divided into two types according to provide electrons : filament tubes , in which source of electrons is a hot filament , and gas tubes in which electrons are produced by ionization of a small quantity of gas in the tube. The x-ray tube is a relatively simple electrical device typically containing two principle elements: a cathode and an anode. As the electrical current flows through the tube from cathode to anode, the electrons undergo an energy loss, which results in the generation of x-radiation. X-ray tube consist from: 1)Anode The anode is the component in which the x-radiation is produced. It is a relatively large piece of metal that connects to the positive side of the electrical circuit. The anode has two primary functions: (1) to convert electronic energy into x-radiation, and (2) to dissipate the heat created in the process. Figure show cross section of an x-ray tube. *The fraction of the total electronic energy that is converted into xradiation (efficiency) depends on two factors: the atomic number (Z) of the anode material and the energy of the electrons. Most x-ray tubes use tungsten, which has an atomic number of 74, as the anode material. In addition to a high atomic number, tungsten has several other characteristics that make it suited for this purpose. Tungsten is almost unique in its ability to maintain its strength at high temperatures, and it has a high melting point and a relatively low rate of evaporation. 2) Focal Spot Not all of the anode is involved in x-ray production. The radiation is produced in a very small area on the surface of the anode known as the focal spot. The dimensions of the focal spot are determined by the dimensions of the electron beam arriving from the cathode. In most x-ray tubes, the focal spot is approximately rectangular. The dimensions of focal spots usually range from 0.1 mm to 2 mm. X-ray tubes are designed to have specific focal spot sizes; small focal spots produce less blurring and better visibility of detail, and large focal spots have a greater heatdissipating capacity. Most x-ray tubes have two focal spot sizes (small and large), which can be selected by the operator according to the imaging procedure. 3)Cathode The basic function of the cathode is to expel the electrons from the electrical circuit and focus them into a well-defined beam aimed at the anode. The typical cathode consists of a small coil of wire (a filament) recessed within a cup-shaped region, as shown below. The filament of the cathode is heated in the same way as a light bulb filament by passing a current through it. This heating current is not the same as the current flowing through the x-ray tube that produces the xradiation. During tube operation, the cathode is heated to a glowing temperature, and the heat energy expels some of the electrons from the cathode. 4) Envelope The anode and cathode are contained in an envelope. The majority of x-ray tubes have glass envelopes, although tubes for some applications have metal and ceramic envelopes. The primary functions of the envelope are to provide support and electrical insulation for the anode and cathode and to maintain a vacuum in the tube. The presence of gases in the x-ray tube would allow electricity to flow through the tube freely, rather than only in the electron beam. This would interfere with x-ray production and possibly damage the circuit. Lec. No. (9) Detection of x-ray 1)Photographic film: is affected by x-ray in much the same way as by visible light ,and film is the most widely used means of recording diffracted x-rays beams. However, the emulsion on ordinary film is too thin to absorb much of the incident x-radiation ,and only absorbed x-rays Can be effective with rather thick layers of emulsion on both sides in order to increase the total absorption . the grain size is also made large for the same purpose . this has the unfortunate consequence that x-ray films are grainy , do not resolve fine detail , and cannot stand much enlargement. 2)fluorescent screen: are made of a thin layer of Zinc sulfide containing a trace of (Ni ) mounted on cardboard backing .under the action of x-rays , this compound fluoresces in the visible region , i.e. emits visible light , in this case yellow light. 3)Ionization devices : measure the intensity of x-ray beams by the amount of ionization they produce in a gas . x-ray quanta can cause ionization just as high –speed electrons can namely , by knocking an electron out of gas molecule and leaving behind a positive ion this phenomenon can be made this basis of intensity measurements by passing the x-ray beam through a chamber containing a suitable gas and two electrodes having a constant potential difference between them. The electrons are attracted to the anode and the positive ions to the cathode and a current is thus produced in an external circuit . in the ionization chamber, this current is constant for constant x-ray intensity , and the magnitude of the current is a measure of the x-ray intensity.








