LCPS Core Experience | Acids and Bases Investigation ONE

LCPS Core Experience | Acids and Bases Investigation ONE
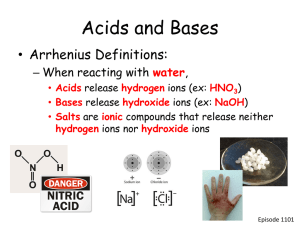
Acids and Bases
S t u d e n t N o t e s
OBJECTIVES
Students will:
recognize some acids and bases as common and familiar household chemicals.
realize that acids and bases are not necessarily strong or dangerous.
determine the pH of different chemical compounds and categorize them as acids or bases.
investigate how the difference between acids and bases correlates to the difference in hydrogen ion concentration of solutions of the two classes of compounds.
LINK
1.
Are all acids and bases strong or dangerous?
YES or NO
2.
What does chemically reactive mean? True or False:
The substances that are involved in the reaction will dissociate or break apart and reform into new substances.
LCPS Core Experience | Acids and Bases Investigation ONE
Background
Acids are a group of chemical compounds that produce hydrogen ions (H
+
) when mixed with water. Ions are atoms or groups of atoms that have either a positive or a negative charge.
Acids are compounds that produce hydrogen ions (H + ) when the acid is mixed with water. The chemical properties of acids result from the production of hydrogen ions
(H
+
) which are very reactive.
For example, hydrochloric acid (HCl), the acid that helps food digestion in the stomach, produces hydrogen ions (H
+
) according to the following equation:
HCl → H
Hydrochloric acid
Notice that producing a hydrogen ion (H
+
Hydrogen ion
+ Cl
─
Chloride ion
+
) also produces a negative ion, the chloride
(Cl
─
) ion.
The amount of anything dissolved in a solution is called its concentration. The concentration of hydrogen ions (H
+
) depends on the amount of acid dissolved. A large amount of dissolved acid results in a large number of hydrogen ions (H + ) in the water.
The concentration of hydrogen ions (H
+
) also depends on the acid. Each molecule of the strong acid hydrochloric acid (HCl) separates into one hydrogen ion (H + ) and a negative chloride (Cl
─
) ion. Each molecule of acetic acid, the weak acid found in vinegar, does not completely separate into a hydrogen ion (H
+
) and a negative ion like hydrochloric acid. This results in a lower concentration of hydrogen ions. (H
+
)
The pH scale allows a scientist to measure the concentration of hydrogen ions (H
+
).
This scale starts at zero which represents a high concentration of hydrogen ions (H
The scale extends to 14, which represents a low concentration of hydrogen ions (H
For example, a solution with a pH of 0 has a higher concentration of hydrogen ions (H
+
).
+ ).
+
) than a solution with a pH of 14. A solution with a pH of 4 has a lower concentration of hydrogen ions (H + ) than a solution with a pH of 3.
Special indicator paper is used to measure the pH of a solution. pH paper has several different chemical compounds added to it that change color when the concentration of hydrogen ions (H + ) is low or high.
LCPS Core Experience | Acids and Bases Investigation ONE
High
H + Ion Concentration
Low
0
Strong
Acid
1 2 3 4 5 6 7
Neutral
pH Scale
8 9 10 11 12 13
Strong
Base
14
STOP AND DISCUSS
EXPERIMENT
1.
In this lesson, you will determine the pH of several different solutions that contain acids.
Lesson One: Observing the pH of Acidic Solutions
2.
Obtain the test tube rack labeled Lesson One.
3.
Using forceps grasp the top of a pH paper and dip it into the vinegar. Remove the pH paper strip.
4.
Match the color(s) of the pH paper with the pictures on the box. Estimate the pH of the vinegar that most closely corresponds to the color(s) in the pictures. Estimate the pH to the nearest half of a pH unit, for example, 3.0, 3.5 or 4.0. Record the pH value in Table A .
5.
Repeat steps 1 through 3 for each of the other acids.
Table A: The pH of Various Solutions
Vinegar
Solution
Compound
Name
Acetic acid
Compound
Formula
H(C
2
H
3
O
2
) pH of
Solution
Automobile Battery Acid Sulfuric acid H
2
(SO
4
)
Stomach Acid
Lemon Juice
Hydrochloric acid
Citric acid H
2
HCl
(C
6
H
7
O
Carbonated Drink Carbonic acid H
2
(CO
3
)
6.
Question: Which of the acids that were tested are familiar to you?
All None _______________________
7)
LCPS Core Experience | Acids and Bases Investigation ONE
7.
Question: Using the data from Table A , write the names of the acids in order from lowest to highest pH . Use the pH Scale from the Background. o
S____________ acid, Hydrochloric acid, A___________acid, Citric acid,
___________________acid
1.
Question: What does the order of the acids tell you about the concentration of H
+ ions in each solution of acid? (Hint: Use the pH scale for help). o The order of the acids shows that the H+ ion concentration ______________ as the pH __________________.
1.
Question: Which atom is present in the chemical formula of each acid? o
__________________is present in each chemical formula.
Background
Bases are a second group of chemical compounds that produce ions when mixed with water. When a base is mixed with water, it produces hydroxide ions (OH
─
) from the paired OH atoms plus a positively charged ion. For example, sodium hydroxide
(NaOH), the base in drain cleaner, produces hydroxide ions (OH
─
) according to the following equation:
NaOH → Na + + OH
─
Sodium Sodium Hydroxide hydroxide ion ion
When a base and an acid are mixed with water, hydroxide ions (OH
─
) react with hydrogen ions (H
+
) to form water which removes hydrogen ions (H
+
) from the solution.
Sodium hydroxide (NaOH) reacts with hydrochloric acid (HCl) according to the following equation:
Na + ion
+ OH
─
Sodium ion
+ H +
Hydroxide Hydrogen ion
+ Cl
─ ion
→ H
2
O + Na +
Chloride Water molecule
Sodium ion
+ Cl
─ ion
Chloride
Hydrogen ions (H
+
) are not a product of the reaction showing that they are removed from the solution and their concentration is decreased. Notice that the other products are water (H
2
O) and Na
+
and Cl
─
ions.
LCPS Core Experience | Acids and Bases Investigation ONE
Background (continued)
The concentration of hydrogen ions (H
+
) also depends on which base is in solution. A strong base like sodium hydroxide always reacts with hydrogen ions (H
+
). The weaker base, magnesium hydroxide, Mg(OH)
2
, that is found in antacid medications, does not always react with hydrogen ions (H
+
) leaving some unreacted base.
The pH scale can also be used to measure the concentration of hydrogen ions (H
+
) in a solution that contains a base. A weak base cannot remove many hydrogen ions (H +) from a solution, so the concentration of hydrogen ions (H
+
) will be higher and the pH will be lower.
A strong base will remove many more hydrogen ions (H
+)
from a solution, so the concentration of hydrogen ions (H + ) will be lower and the pH will be higher.
Refer to the pH scale earlier in the investigation.
STOP AND DISCUSS
Lesson Two: Observing the pH of Basic Solutions
1.
In this Lesson, you will determine the pH of several different solutions that contain bases.
2.
Obtain a test tube rack labeled Lesson Two.
3.
Using forceps grasp the top of the pH paper strip and dip it in the drain cleaner. Remove the pH paper strip.
4.
Match the color(s) of the pH paper strip with the pictures on the box. Estimate the pH of the drain cleaner that most closely corresponds to the color in the pictures. Estimate the pH to the nearest half of a pH unit, for example, 3.0, 3.5 or 4.0. Record the pH value in Table B .
5.
Repeat steps 1 through 3 for each of the other bases.
Table B: The pH of Various Solutions
Solution
Drain Cleaner
Chemical
Compound
Sodium hydroxide
Chemical
Formula
NaOH pH of
Solution
Window Cleaner Ammonium hydroxide NH
4
OH
Antacid Medication Magnesium hydroxide Mg(OH)
2
LCPS Core Experience | Acids and Bases Investigation ONE
6.
Question: Which of the bases that were tested are familiar to you?
All None ____________________________
7.
Question: Write the names of the bases in order from lowest to highest pH. Use the pH Scale from the Background.
M___________________ Hydroxide, Ammonium Hydroxide, _______________ Hydroxide
8.
Question: What does the order of the bases tell you about the concentration of hydrogen ions
(H + ) in each solution of base?
The order of the bases shows that the H+ concentration _____________as the pH increases.
9.
Question: Which two elements are present in the chemical formula of each base?
Oxygen and ________________ are present in each chemical formula .
LEARNING REVIEW
List three things you have learned about acids, bases, hydrogen ion concentration and pH from this investigation. a.
When an acid is in a solution with water, the acid dissociates into one or more hydrogen
(H
+
) ions. Hydrogen ions are _______________ in the solution. b.
When a base is in a solution with water, the base dissociates into one or more hydroxide
(OH ) ions. Hydrogen ions are ________________ from the solution. c.
The higher the concentration of hydrogen ions (H
+
) in the solution, the stronger the
__________. The lower the concentration of hydrogen ions (H
+
) in the solution, the stronger the _____________.
STOP AND DISCUSS
LCPS Core Experience | Acids and Bases Investigation ONE
EVALUATION
1.
Why are the pH values of the substances in Lesson One all in the acidic range? What happened to the H
+ ion concentration in this lesson?
The pH values are all in the acidic range because the substances are all __________ which produce H + ions in water solution.
2.
Why are the pH values of the substances in Lesson Two all in the basic range? What happened to the H
+ ion concentration in this lesson?
The pH values are all in the basic range because the substances are all ____________ which remove H + from water solution .
3.
What is the relationship between a low pH and the hydrogen ion (H
+
) concentration?
A __________ pH corresponds to a __________ H
+
ion concentration.
4.
What is the relationship between a high pH and the hydrogen ion (H
+
) concentration?
A __________ pH corresponds to a __________ H
+
ion concentration.
5.
What properties make acids and bases chemically reactive?
_____________ release H
+
ions when they are mixed with water and ______________ remove H
+
ions when they are mixed with water.
6.
What is the relationship between the concentration of hydrogen ions (H + ) in a solution and the chemical reactivity of strong acids and weak acids?
_________________ acids are able to release more H
+
ions when mixed with water resulting in a higher concentration of H
+
ions. _______________ acids release fewer H
+
ions resulting in a lower concentration of H
+
ions.
7.
You are a lifeguard at a community pool and take a pH test of the pool water. The pH reading is 8. Explain what you would add to the water so that the pH reading is neutral?
You would need to add __________tablets to the pool water to lower the pH to 7, which is the pH of neutral water.