chemistry_assessment_-_1st_quarter_revised
advertisement

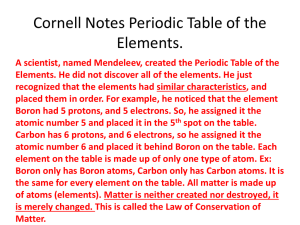
Chemistry Assessment – 1st Quarter 1) What element would have one more valence electrons than phosphorus A) Nitrogen B) Calcium C) Gold D) Selenium 2) How many valence electrons are present in an Iodine ion A) 5 B) 10 C) 7 D) 8 3) The element Potassium (K) has how many electrons in each energy A) 1st 2, 2nd 8, 5th 8 B) 1st 2, 2nd 8, 3rd 8, 4th 1 C) 1st 2, 2nd 2, 3rd 8, 4th 7 D) 1st 2, 2nd 6, 3rd 9, 4th 2 4) Which of the following parts of the periodic table best describes the outermost energy level for an element? A) Periods B) Groups C) Families D) Columns 5) Rank the following sections of the periodic table from most to least reactive A) Alkali Metals, Transition Metals, Alkaline Earth Metals, Noble Gases B) Alkali Metals, Alkaline Earth Metals, Transitional Metals, Noble Gases C) Transition Metals, Alkaline Earth Metals, Alkali Metals , Noble Gases D) Noble Gases, Alkaline Earth Metals, Transition Metals, Alkali Metals 6) In general what happens to the reactivity of an nonmetal as you move down a group or column A) No clear trend exists B) The Reactivity increases C) The Reactivity decreases D) There is no change 7) Why do elements in the lower right hand corner of the periodic table have the highest densities A) As you move down the periodic table atomic radius increases B) As you move down the periodic table atomic mass increases C) As you move from left to right in the periodic table atomic radius decreases D) Both b and C 8) In general what happens to the melting point of elements in Group 16 in the periodic table? A) As you move down the period it increases B) As you move down the period decreases C) the melting point for all Group 16 elements is the same D) none of the above 9) Using the elements Copper (Cu), Germanium (Ge), and Chlorine (Cl) which is electrically a nonconductor, a semiconductor, and a conductor? A) Cu is a conductor, Ge is a non conductor, and Cl is a semiconductor B) Cu is a conductor, Ge is a semiconductor, and Cl is a nonconductor C) Cu is a semiconductor, Ge is a conductor, and Cl is a nonconductor D) Cu is semiconductor, Ge is a nonconductor, and Cl is a semiconductor 10) What happens when a covalent bond is forming? A) Electrons are shared B) Electrons are taken C) Electrons are given D) Electrons are neither transferred nor shared 11) What happens when an ionic bond is forming? A) Electrons are shared B) Electrons are taken C) Electrons are received D) Both B and C Match the correct letter with its Family (Group). 12.___________ Halogens 13.___________ Alkaline Earth Metals 14.___________ Transition Metals 15.___________ Noble Gases 16.___________ Inner Transition Metals 17.___________ Alkali Metals 18) Which of the following pairs of elements will react similarly? A) sodium & helium B) nitrogen & potassium C) magnesium & barium D) hydrogen & oxygen 19) Which of the following is not a property of an ionic compound? A) strong electrolyte B) low boiling point C) crystalline structure D) high melting point 20) Which of the following pairs of elements would form a covalent bond? A) carbon and chlorine B) potassium and chlorine C) iron and oxygen D) sodium and bromine 21) Which of the can be separated by physical means? A) element B) compound C) pure substance D) mixture 22) Which of the following is not a pure substance? A) mercury B) distilled water C) copper (II) chloride solution D) carbon dioxide Use the following to answer questions 23 - 25 A. K+ B. Hg C. NO2 D. MgSO4 23) Which of the above is a molecule? _____________ 24) Which is an atom? _________________ 25) Which is a formula unit? ________________ 26) Which is an ion?_____________ 27) Which of the following is the correct symbol for an aluminum ion? A) Al3+ B) Al2+ C) Al+ D) Al 28) How do isotopes of an element differ from one another? A) number of protons B) number of electrons C) number of neutrons D) number of protons and neutrons 29) Which of the following pairs of atoms have the same number of electrons? A) O2- and Ne B) K+ and Li+ C) Fe3+ and Fe2+ D) N3- and K+ Use the following to answer question 30. 1. atomic number 5, mass number 15 2. atomic number 6, mass number 15 3. atomic number 5, mass number 16 4. atomic number 4, mass number 16 30. Which two of the above are isotopes? A) 1 & 2 B) 1 & 3 C) 2 & 3 D) 3 & 4 Answer Key 1) D 2) C 3) B 4) A 5) B 6) C 7) D 8) A 9) B 10)A 11)D 12)D 13)B 14)C 15)D 16)F 17)A 18)C 19)B 20)A 21)D 22)C 23)C 24)B 25)D 26)A 27)A 28)C 29)A 30)B