Predicting Products of a Reaction
advertisement

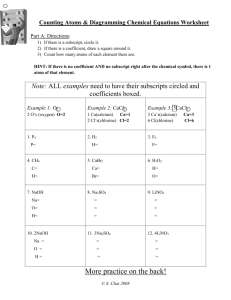
Name _______________________________________ Chemistry Mr. Harper CH 8: Chemical Reactions Study Guide 1. What is a chemical reaction? a. What are the signs that a chemical reaction has occurred? b. How do you know for sure that a chemical reaction has occurred? c. What happens to atoms during a chemical reaction? d. What happens to energy during a chemical reaction? i. What happens when bonds form? When they break? ii. What is an exothermic reaction? Endothermic? iii. What is activation energy? iv. How do you interpret an energy graph for a reaction? e. What are four factors that can affect the rate of a reaction? 2. How do you represent a chemical reaction? a. What is a reactant? What is a product of a reaction? b. How and why must you balance a chemical equation? What numbers can you change and which can you not change? c. What are the symbols used to indicate certain conditions for reactions to occur? d. What are the diatomic elements? 3. What are the 5 types of chemical reactions and their general equations? a. How can you predict the products of simple chemical reactions? b. How can you predict the states of matter for substances in a chemical reaction? c. What is the activity series used for? d. How do you use the solubility rules? e. What type of reaction usually needs to be heated? f. What type of reaction usually releases heat? g. What are the products when a hydrocarbon is combusted? Bookwork 1. p.268 #1-3 2. p.274 #1 (Practice); p.274 #1-5 (Section Review) 3. p.284 Quick Lab and #1-5 (Section Review) Iron and Sulfur Reaction Introduction: In this lab, you will carry out a reaction between iron and sulfur to examine what happens during a typical chemical reaction. Materials: Iron powder Sulfur powder Watch glass Magnet Paper towel 2 weigh boats TBB Fume hood Procedure: 1. Obtain a weigh boat and measure out 1.75 g of iron powder. 2. Obtain another weigh boat and measure out 1.00 g of sulfur powder. 3. Observe and note the appearance and properties of these two substances. 4. Mix the two powders by pouring repeatedly from one weigh boat to the other until a homogeneous mixture (by appearance) is obtained. 5. Cover the end of a magnet with a paper towel and dip it into the mixture. Can the mixture be separated by physical means? How? 6. Return the contents on the magnet to the mixture and remix until homogeneous. 7. Pour the mixture onto a watch glass and measure and record the mass of the mixture and watch glass. 8. Bring the watch glass and mixture to the fume hood. 9. Follow the teacher’s instructions at the fume hood. Record your observations. 10. Allow the watch glass and mixture to cool. Then, measure and record the mass again. 11. Observe and note the appearance and properties of the product formed by the reaction. 12. Cover the end of a magnet with a paper towel and touch it to the product. Can the product be separated by physical means? Is it magnetic? 13. Discard the product in the trash can, clean up your lab station and equipment, and wash your hands. Analysis: 1. What are the reactants of this reaction? What are the products? 2. What are the properties of the reactants? What are the properties of the product? 3. Write the balanced chemical equation for this reaction. What type of reaction is this? 4. What were the signs that a chemical reaction occurred? How do you know for sure that a chemical reaction occurred? 5. What changes occurred as a result of the reaction? 6. What happened to the total mass during the reaction? Is there any discrepancy with scientific laws? Explain why or why not. 7. Why did the reactants need to be heated for the reaction to occur? 8. What changes in energy took place throughout the reaction? 9. Is energy usually absorbed or released when chemical bonds are broken? What about when bonds are formed? 10. What happened to the valence electrons of the atoms during the reaction? 11. What would the result of this reaction be if the reactants were not mixed thoroughly? Explain your answer. 12. Which observations that you made were qualitative and which were quantitative? Lab Report: Organize your data and observations from the lab. Present them neatly in a lab report along with the answers to the analysis questions. Types of Reactions Comparison Synthesis Decomposition Single Displacement Double Displacement Combustion Synthesis Reactions Lab activity: 1. Put on your goggles and apron. 2. Place a strip of magnesium in the flame of the Bunsen burner. Do not look directly at the flame. Once it has reacted, place it on a watch glass to analyze the products. Note your observations and write the balanced chemical equations. Use pp.276-279 in the textbook to help you. 3. Using a pair of tongs, hold a piece of steel wool in the flame of the Bunsen burner until all the wool has reacted. Place the burnt steel wool on the lab table for analysis. Note your observations and write the balanced chemical equation. 4. Wet a piece of steel wool and place it on a watch glass. Using a piece of tape, put your names on the beaker and set it aside until tomorrow. Note your observations and write the balanced chemical equation. 5. Measure out 25 mL of water and place it in a small beaker. Add a few drops of phenolphthalein solution. Place a small piece of calcium oxide in the water. Note your observations, and write the balanced chemical equation. Practice: Write balanced chemical equations for the following reactions. Include all symbols and states of matter. Use pp.276-279 in the textbook to help you. 13. Zinc reacts with sulfur. 14. Carbon is burned in air. 15. Carbon is burned in air with a limited supply of oxygen. 16. Potassium reacts with iodine. 17. Sodium oxide is placed in water. Decomposition Reactions Lab activity: 1. Put on your goggles and apron. 2. Put two small scoops of potassium chlorate into a test tube. Note the appearance of the sample. Using a test tube clamp, heat the potassium chlorate over the Bunsen burner until in melts. Aim the mouth of the test tube away from yourself and others. After it melts, turn the Bunsen burner off. Then, light a match and carefully place it in the test tube. Note all observations, and write the balanced chemical equation. 3. Put 50 mL of water in a small beaker. Add a couple scoops of table salt and swirl it around to dissolve it. Construct a simple circuit with a light bulb and immerse the leads in the solution. Note all observations, and write the balanced chemical equation. Practice: Write balanced chemical equations for the following reactions. Include all symbols and states of matter. Use pp.279-280 in the textbook to help you. 1. Mercury (II) oxide is heated. 2. Barium carbonate is heated. 3. Solid magnesium hydroxide is heated. 4. Lithium chlorate is heated. 5. Sulfurous acid is heated. Single Displacement Reactions Lab activity: 1. Put on your goggles and apron. 2. Put approximately 5 mL of HCl acid in a test tube (about ½ inch high). Add a piece of magnesium. Note all observations and write the balanced chemical equation. 3. Put approximately 5 mL of HCl acid in another test tube (about ½ inch high). Add a piece of copper. Note all observations and write the balanced chemical equation. Practice: Write balanced chemical equations for the reactions below. Include all symbols and states of matter. Use pp.281-282 & 285-287 as a guide. 1. Zinc is mixed with aqueous lead (II) nitrate. 2. Chlorine gas is added to a solution of lithium fluoride. 3. Potassium is placed in water. 4. Mercury is mixed with aqueous lead (II) nitrate. 5. Chlorine gas is added to a solution of lithium bromide. 6. Read pp. 285-287 and complete the practice (#1-3) and section review (#1-4) on p. 287. Activity Series: Double Displacement Reactions Lab activity: 1. Put on your goggles and apron. 2. Put about 5 mL of silver nitrate in a test tube. Add a couple drops of sodium chloride solution. Note all observations and write the balanced chemical equation. 3. Put about 5 mL of lead (II) nitrate in a test tube. Add a couple drops of potassium iodide solution. Note all observations and write the balanced chemical equation. 4. Put about 5 mL of HCl acid in a test tube. Add a drop of universal indicator. Put 5 mL of potassium hydroxide in another test tube. Add a drop of universal indicator. Pour the HCl acid into the potassium hydroxide test tube. Note all observations and write the balanced chemical equation. 5. Put a small scoop of baking soda in a test tube. Add a couple drops of acetic acid. Note all observations and write the balanced chemical equation. Practice: Write balanced chemical equations for the reactions below. Include all symbols and states of matter. Use pp.282-283 as a guide. 1. Sulfuric acid is mixed with aqueous barium hydroxide. 2. Solutions of silver nitrate and sodium chloride are mixed. 3. Solutions of magnesium nitrate and potassium hydroxide are mixed. 4. Solutions of lithium hydroxide and iron (III) nitrate are mixed. Combustion Reactions Lab activity: Watch the demonstrations with the Bunsen burner, lighter fluid, and the gummy bear. Note all observations and write the balanced chemical equations for all three reactions. Use pp.283 as a guide. 1. Bunsen burner, combustion of methane (CH4): 2. Combustion of lighter fluid (C5H12): 3. Combustion of gummy bear (glucose: C6H12O6): Practice: Write balanced chemical equations for the reactions below. Include all symbols and states of matter. 1. Butane (C4H10) is ignited in air. 2. Octane (C8H18) is ignited in air. 3. Ethene (C2H4) is ignited in air. 4. Ethanol (C2H5OH) is combusted. Rate of Reactions Lab Lab activity: 1. Split a denture tablet in half. Put one half in a beaker with 50.0 mL of water. Observe what happens. Next, crush the other half into a fine powder and put it into another beaker with 50.0 mL of water. Compare the rates of the two reactions and come up with a theory for why the one happens faster. 2. Measure out 1.0 g of sugar. Place the sugar in a beaker with 50.0 mL of cold water and swirl it around to dissolve the sugar. Repeat this procedure except this time use hot water. Compare the rates of the sugar dissolving and come up with a theory for why the one happens faster. 3. Pour 3 mL (about an inch high in the test tube) of hydrogen peroxide into a test tube. Record anything that happens to it during the lab time. Pour 3 mL of hydrogen peroxide into another test tube and add a dropper full of potassium iodide solution. Observe what happens. Compare the rates of the two reactions and come up with a theory for why the one happens faster. (Hint: What is the potassium iodide acting as?) 4. Watch the demonstration with zinc and dilute hydrochloric acid versus concentrated hydrochloric acid. Observe what happens. Compare the rates of the two reactions and come up with a theory for why the one happens faster. 5. Identify and explain four things that can affect the rate of a reaction. Identifying and Balancing Chemical Reactions Identify whether the following reactions are decomposition, synthesis, single displacement, double displacement, or combustion. Then balance the chemical reaction. Type of Reaction 1. ____ Al + ____ H2SO4 ____ Al2(SO4)3 + ____ H2 ___________________________ 2. ____ C3H8 + ____ O2 ____ CO2 + ____ H2O ___________________________ 3. ____ NaClO3 ____ NaCl + ____ O2 ___________________________ 4. ____ KOH ____ K2O + ____ H2O ___________________________ 5. ____ Cu + ____ AgNO3 ____ Cu(NO3)2 + ____ Ag ___________________________ 6. ____ (NH4)2SO4 + ____ AgNO3 ____ Ag2SO4 + ____NH4NO3_________________________ 7. ____ Li + ____ O2 ____ Li2O ___________________________ 8. ____ AuCl ____ Au + ____ Cl2 ___________________________ 9. ____ C12H22O11 + ____ O2 ____ CO2 + ____ H2O ___________________________ 10. ____ Ba + ____ H2O ____ BaO + ____ H2 ___________________________ 11. ____ Pb(NO3)2 + ____ NaOH ____ Pb(OH)2 + ____ NaNO3 __________________________ Predicting Products of a Reaction (I) This worksheet is designed to help you predict products of simple reactions of the five basic reaction types (synthesis, decomposition, single replacement, double replacement, and combustion reactions. For the first few reactions, the type of reaction is listed, you should predict the products, then balance. Further questions just have the reactants listed and you should decide on the type of reaction, as well as the correct products. Although states (s, l, g, aq) of the reactants and products are very important in a chemical reaction, don’t worry about determining those for these problems. Rather, focus on what products might result from the reactants given. a. Combustion: C6H12 + O2 b. Combustion: C4H6 + O2 c. Combustion: C6H10O3 + O2 I2 1. Synthesis: Mg + 2. Double displacement: CuCl2 + H2S 3. Double displacement: NaOH + HClO4 4. Decomposition: ZnCO3 + heat 5. Single replacement: HCl + Zn 6. ________________ Na + MgCl2 7. ________________ CaCl2 + K2CO3 + K 9. ________________ BaCl2 + K3PO4 10. ________________ H2SO4 + KOH 11. ________________ Al2(CO3)3 + heat 12. ________________ Al O2 13. ________________ Pb(NO3)2 + KOH 14. ________________ C2H2 + O2 15. ________________ Ca + AgCl 16. ________________ C3H8 + O2 17. ________________ Li + N2 18. ________________ HCl + Mg(OH)2 19. ________________ Mg(OH)2 + heat 20. ________________ Fe(OH)3 + heat + Cl2 8. ________________ Predicting Products of a Reaction (II) Identify the type of reaction (or no reaction), predict the products of the reaction from the given reactants, and write the balanced chemical equation including states of matter and symbols. 1. Solid cesium hydroxide is added to a solution of zinc acetate. 2. Silver metal reacts with chlorine gas. 3. Solid magnesium carbonate is heated. 4. Propane gas (C3H8) is burned in air. 5. Nickel metal is added to a solution of aluminum nitrate. 6. Ethanol (C2H5OH) is mixed with oxygen gas with sufficient heat to begin the reaction. 7. Hydrochloric acid is neutralized with aqueous sodium hydroxide. 8. Zinc is added to a solution of lead (II) nitrate. 9. Solid sodium chlorate is heated. 10. Iron is mixed with sulfur and heated. Predicting Products of a Reaction (III) Identify the type of reaction (or no reaction), predict the products of the reaction from the given reactants, and write the balanced chemical equation. Include states of matter and symbols. 1. Zinc metal is mixed with steam. 2. Solid cobalt (II) chloride is added to a solution of silver nitrate. 3. Solutions of ammonium carbonate and potassium bromide are combined. 4. Iron metal is added to a solution of magnesium chloride. 5. Chlorine gas is bubbled through a solution of calcium bromide. 6. Liquid bromine is added to a solution of ammonium chloride. 7. Methanol (CH3OH) is mixed with oxygen gas and ignited. 8. An electric current is run through a beaker of water. 9. Solid copper (I) oxide is heated. 10. Hydrogen gas is mixed with oxygen gas with sufficient heat to begin the reaction. Predicting Products of a Reaction (IV) Identify the type of reaction (or no reaction), predict the products of the reaction from the given reactants, and write the balanced chemical equation including states of matter and symbols. 1. Aqueous lithium hydroxide and aqueous sodium chromate are mixed together and heated. 2. Copper metal is added to a solution of hydrobromic acid. 3. Butane (C4H10) is mixed with oxygen gas and ignited. 4. Hydroidic acid is neutralized with lithium hydroxide. 5. Solid sodium carbonate is heated. 6. Octane (C8H18) is mixed with oxygen gas and ignited. 7. Cadmium metal is mixed with steam. 8. Strontium metal is mixed with liquid bromine. 9. Carbon is burned in air with an excess of oxygen. 10. Solid potassium chlorate is heated.