Birthplace
advertisement

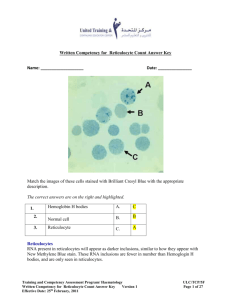
Reticulocyte Count Date of SC final approval About the Measure Domain: Measure: Definition: Sickle Cell Disease: Cardiovascular, Pulmonary, and Renal Reticulocyte Count A bioassay to measure the number of reticulocytes (immature red blood cells). Purpose: Reticulocyte count measures how fast red blood cells are made. Elevated reticulocyte counts are associated with blood loss, hemolysis and hemolytic anemia, and in response to the anemia of clinical conditions such as sickle cell disease. Reticulocyte counts can also be used to measure efficacy of treatments (e.g., iron replacement therapy). About the Protocol Description of Protocol: Selection Rationale: Specific Instructions: This protocol provides instructions for drawing, processing and storing blood according to the National Health and Nutrition Examination Survey (NHANES) methods. Because there are many comparable assays and instruments for measuring reticulocyte count, the protocol also provides basic guidelines to aid comparability among different studies. The Sickle Cell Disease Working Group 1 (Cardiovascular, Pulmonary, and Renal) selected the National Health and Nutrition Examination Survey 2011-2012 protocol as the best standardized methodology for blood collection, processing and storage. The National Health and Nutrition Examination Survey (NHANES) instructions for drawing, processing, and storing blood provide a standard methodology used successfully for many years to ensure comparable results across study sites. However, the Sickle Cell Disease Working Group 1 (Cardiovascular, Pulmonary, and Renal) notes that certain aspects (e.g., exclusion criteria) of the NHANES protocol are study specific and might not be applicable to all types of studies (e.g., sickle cell disease). Reticulocyte count can be combined with other indirect markers of hemolysis (haptoglobin level, bilirubin level, red blood cell aspartate aminotransferase level, and lactate dehydrogenase level) to derive a hemolytic component for sickle cell disease patients. Collection of blood samples for the measurement of analytes requires a general determination of whether to use serum or plasma for the assay and also a determination of the type of collection tube to be obtained. For example, if serum is to be used a determination needs to be made as to whether red top or serum gel separator collection tubes are used. While comparable values are obtained for many analytes from either serum or plasma, there may be situations where differences are more pronounced and serum or plasma specific norms will be needed for references. The NHANES protocol presented here uses red top/serum separator tubes. At times it may be possible to collect both, but other considerations such as participant burden may be the deciding factor. It is important to match assay type with sample type. Some automated devices may preclude the use of serum, for example, while others may be optimized for it. Investigators should choose methods of collection that match the methods of analysis. This will best be done by communicating with the laboratory where the proposed assays will be performed. They will become an Version 10 – 10/21/09 Reticulocyte Count Protocol Text: Date of SC final approval important partner with you in ensuring that there is compatibility from collection to assays to interpretation and reporting of levels and results The following is a summary version of the full National Health and Nutrition Examination Survey 2011-2012 protocol. Exclusion Criteria Persons will be excluded from this component if they: • Report that they have hemophilia; or • Report that they have received cancer chemotherapy in the last 4 weeks SP = Sample Person. 1. Do you have hemophilia? 1[] 2[] 7[] 9[] Yes No Refused Don’t Know If the SP answers "Yes," the SP is excluded from the blood draw. If SP answers "No" or "Don’t Know," blood is drawn from the SP. 2. Have you received cancer chemotherapy in the past four weeks or do you anticipate such therapy in the next four weeks? 1 [ ] Yes 2 [ ] No 7 [ ] Refused 9 [ ] Don’t Know If the SP answers "Yes," the SP is excluded from the blood draw. If SP answers "No" or "Don’t Know," blood is drawn from the SP. Venipuncture Procedures Editor’s Note: Please review chapter 4 of the Laboratory Procedures Manual from the 2011-2012 National Health and Nutrition Examination Survey (NHANES) for a full description of phlebotomy procedures. This manual is posted here, and is also available at the NHANES website: 2011-2012 NHANES Laboratory Procedures Manual Venipuncture should generally be performed using the median cubital, cephalic, or basilic veins in the left arm unless this arm is unsuitable. If the veins in the left arm are unsuitable, look for suitable veins on the right arm. If the veins in the antecubital space on both arms are not suitable, then look for veins in the forearm or dorsal side of the hand on the left arm/hand and then the right arm/hand. Recording the Results of the Venipuncture Procedure Version 10 – 10/21/09 Reticulocyte Count Date of SC final approval Immediately after completing the venipuncture, record the results of the blood draw, the reasons for a tube not being drawn according to the protocol, and any comments about the venipuncture. Blood Processing Please review chapter 8 of the Laboratory Procedures Manual from the National Health and Nutrition Examination Survey 2011-2012 for a full description of blood processing procedures: 2011-2012 NHANES Laboratory Procedures Manual • • • Allow the blood to clot by setting aside for 30 to 45 minutes at room temperature. Do not clot for more than an hour. Centrifuge the tube at room temperature to separate the serum and aliquot into an appropriate storage tube. Determine if the serum is hemolyzed, turbid, lipemic, or icteric. If so, enter a comment to describe the serum. Laboratory Assay for Reticulocyte Count The Sickle Cell Disease Working Group 1 (Cardiovascular, Pulmonary, and Renal) notes that there are a number of different assays and instruments that are appropriate to measure the reticulocyte count from serum. Once an assay is chosen for a particular study, the Working Group recommends that no changes in the protocol be made over the course of the study. To aid comparability, the Working Group recommends that the investigator record the make and manufacturer of equipment used and the repeatability and coefficients of variation for the assay. Reference Ranges for Reticulocyte Count: Children: 3 - 6% Adults: 0.5 - 1.5% Percentage of reticulocytes and absolute reticulocyte counts (ARC) are given by the following formulas: Reticulocyte Percentage = [Number of Reticulocytes / Number of total Red Blood Cells] X 100 Participant: Source: Language of Source: Absolute reticulocyte count (thousands/µL) = reticulocyte % x RBC count (millions/µL) x 10 Individuals age 1 year and older. Centers for Disease Control and Prevention (CDC). (2011). National Health and Nutrition Examination Survey Questionnaire, Laboratory Procedures Manual. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. English Personnel and Training Required: Equipment Needs: Phlebotomist Protocol Type: Bioassay Laboratory with the ability to perform the reticulocyte count assay. Version 10 – 10/21/09 Reticulocyte Count Date of SC final approval Requirements: Requirements Category Common Data Elements: General References: Required (Yes/No): Major equipment No Specialized training No Specialized requirements for biospecimen collection Average time of greater than 15 minutes in an unaffected individual TBD by PhenX Team No No Potoka, K. P., & Gladwin, M. T. (2015). Vasculopathy and pulmonary hypertension in sickle cell disease. American Journal of Physiology, Lung Cellular and Molecular Physiology, 308, L314-L324/ Nouraie, M., Lee, J. S., Zhang, Y., Kanias, T., Zhao, X., Xiong, Z., Oriss, T. B., Zeng, Q., Kato, G. J., Gibbs, J. S., Hildesheim, M. E., Sachdev, V., Barst, R. J., Machado, R. F., Hassell, K. L., Little, J. A., Schraufnagel, D. E., Krishnamurti, L., Novelli, E., Girgis, R. E., Morris, C.R., Rosenzweig, E. B., Badesch, D. B., Lanzkron, S., Castro, O. L., Goldsmith, J. C., Gordeuk, V. R., Galdwin, M. T., & Walk-PHASST Investigators and Patients. (2013). The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica, 98(3), 464-472. doi: 10.3324/haematol.2012.068965 Sachdev, V., Kato, G. J., Gibbs, J. S., Barst, R.J., Machado, R.F., Nouraie, M., Hassell, K. L., Little, J. A., Schraufnagel, D. E., Krishnamurti, L., Novelli, E. M., Girgis, R. E., Morris, C. R., Rosenzweig, E. B., Badesch, D. B., Lanzkron, S., Castro, O. L., Taylor, J. G. 6th, Hannoush, H., Goldsmith, J. C., Gladwin, M. T., Gordeuk, V. R., & Walk-PHASST Investigators. (2011). Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation, 124(13), 1452-1460. Additional Information About the Measure Essential Data: Current age, Race, Ethnicity Related PhenX Measures: Liver Function Assay, Cell Free Hemoglobin, Red Blood Cell Microparticles, Haptoglobin, Lactate dehydrogenase, Red Blood Cell Aspartate Aminotransferase, Bilirubin Hemolytic Component: Derived Variables: Reticulocyte count can be combined with other indirect markers of hemolysis (haptoglobin levels, bilirubin levels, red blood cell aspartate aminotransferase levels, and lactate dehydrogenase levels) by principle component analysis (PCA) to derive a hemolytic component for sickle cell disease patients. Version 10 – 10/21/09 Reticulocyte Count Date of SC final approval Nouraie, M., Lee, J.S., Zhang, Y., Kanias, T., Zhao, X., Xiong, Z., Oriss, T.B., Zeng, Q., Kato, G. J., Gibbs, J. S., Hildesheim, M. E., Sachdev, V., Barst, R. J., Machado, R. F., Hassell, K. L., Little, J. A., Schraufnagel, D. E., Krishnamurti, L., Novelli, E., Girgis, R. E., Morris, C.R., Rosenzweig, E. B., Badesch, D. B., Lanzkron, S., Castro, O. L., Goldsmith, J. C., Gordeuk, V. R., Galdwin, M. T., & Walk-PHASST Investigators and Patients. (2013). The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica, 98(3), 464-472. Keywords/Related Reticulocyte count, hemolysis, hemoglobin, sickle cell disease, SCD, anemia, Concepts: hypoxemia, hemolytic anemia, pulmonary hypertension, chronic kidney disease, CKD, vasculopathy, stroke, rare genetic conditions, hemolytic component Version 10 – 10/21/09