100 μg mL −1 - Springer Static Content Server
advertisement

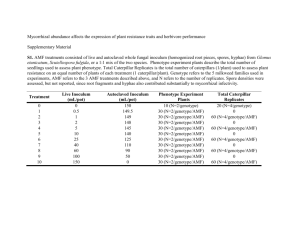
Quantification of AMF root colonization by real-time PCR, using species-specific primers for rLSU Article title: Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species, Rhizophagus irregularis Journal: Mycorrhiza Authors : Sarah Symanczik, Pierre-Emmanuel Courty, Thomas Boller, Andres Wiemken, Mohamed N. Al-Yahya'ei Corresponding author: Sarah Symanczik; Department of Soil Sciences, Research institute of organic agriculture, Ackerstrasse 113, 5070 Frick, Switzerland; sarah.symanczik@fibl.org Methods RNA isolation and quantitative reverse transcription-PCR (qRT-PCR) Roots were ground in liquid nitrogen and total RNA was isolated using the RNeasy Plant Mini kit (Qiagen, Darmstadt, Germany). The DNA-free set (Ambion, Austin, USA) was used to digest DNA after RNA purification. Quantification of transcript numbers of large ribosoma subunit (rLSU) genes was performed using a two-step qRT-PCR procedure. Total RNA was measured with a spectrophotometer (Nanodrop ND-1000, Witec, Switzerland) and then reverse-transcribed (100 ng per reaction) using the iScript cDNA Synthesis kit (Bio-Rad, Paolo Alto, CA, USA). cDNAs were used as templates in qRT-PCR reactions with gene-specific primers designed using Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and amplify 3.1 (http://engels.genetics.wisc.edu/amplify) (Table 1). Design of qRT-PCR markers Primers were designed for specific detection and quantification of Diversispora aurantia, Diversispora omaniana, Septoglomus africanum, an undescribed Paraglomus sp. and Rhizophagus irregularis BEG-75 based on sequencing data published previously (Symanczik et al. 2014a; Symanczik et al. 2014b). Primers were targeting species-specific motifs in the rLSU genes and were tested on cDNA synthesized from AMF spores as well as mycorrhized S. bicolor roots cultivated separately with the different AMF species. cDNA was subjected to PCR amplification of the rLSU genes as previously described (Symanczik et al. 2014a; Symanczik et al. 2014b) using designed primers. The thermal cycling conditions were modified only: initial denaturation at 94°C for 3 min, 30 cycles of denaturation at 94°C for 30s - annealing at 55°C for 30s- elongation at 72°C for 60 s, and for final elongation at 72°C for 5 min. Amplified DNA fragments were cloned into pGEM-T Vector system (Promega, Madison Wisconsin) and further transformed into competent JM109 Escherichia coli cells (Promega, Madison Wisconsin) following the manufacturers’ instructions. Plasmids were isolated from overnight cultures of the transformed E. coli JM109 (Promega), grown on LB medium supplemented with ampicillin (100 μg mL −1) using the ZR Plasmid MiniprepTM -Classic kit (ZYMO Research, Irvine, California). The identity of isolated plasmids were confirmed with direct colony PCR technique using the universal M13F and M13R vector primers and purified with ExoSAP-IT (GE Healthcare, Glattbrugg, Switzerland). For sequencing of plasmids the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster 205 City, California) and the ABI3500 were used. The sequences were individually edited and the identity of the sequences was confirmed by alignment with the full rLSU sequence of each AMF strain. The concentration of the plasmids was estimated with a spectrophotometer (Nanodrop ND-1000, Witec, Switzerland). The plasmids were linearized using Ncol-HFTM digestion (New England BioLabs®, Ipswich, MA, USA), incubating the samples at 37°C for 2 h. The concentration of plasmid copies per unit of sample volume was calculated according to Jansa et al. (2008), by knowing the concentration of DNA in each sample, length of insert (127 bp for S. africanum, 126 bp for D. aurantia, 79 bp for D. omaniana, 71 bp for Paraglomus sp. and 76 bp for R. irregularis), and length of vector (3000 bp), and by assuming molecular weight per nucleotide double-stranded DNA to be 660 Da. Plasmid preparations were serially diluted (10-fold) to achieve a brought range of plasmid concentrations. These were used as templates for generating calibration curves for the conversion of the qRT-PCR detection cycle to rLSU transcript numbers of the AMF species in root samples in an absolute quantification assay (Online resource 2). The primers were confirmed to amplify their target AMF species and to avoid cross-targeting of AMF species as well as sorghum DNA (tested on spores and colonized roots of S. bicolor). Cross-amplification and dilution tests were performed using the diluted plasmid preparations as described above to confirm either the species-specificity of the primer-pairs (selection was based either on no cross-amplification with other AMF species or on a Cp value difference of more than ten cycles between target and non-target species) or the effectively of the primer-pairs (correct amplification even if target template is present at low concentrations). qRT-PCR measurements All qRT-PCR measurements were carried out in 10 μL reaction formats, a LightCycler® 480 System and with Roche SYBR green MasterMix (Roche Diagnostics AG, Rotkreuz, Switzerland). After optimization of the cycling conditions using different concentrations of the primers, the final concentrations in the master mix were 0.4 µM and 0.6 µM for Paraglomus sp. and the other four AMF species, respectively. Cycling conditions were the following: initial denaturation at 95°C for 5 min followed by 45 cycles with denaturation at 95°C for 10s, annealing at 60°C for 10s and elongation at 72°C for 10s. Subsequently melting curves were analyzed to confirm the length of amplified DNA fragments and the absence of primer-dimers and non-specific amplification. Table1 List of PCR primers designed for qRT-PCR analysis AMF species Primer designation Primer sequence Septoglomus G17fw4 TCTGATGGGTCCTACTTATC G17rv4 CGTATCTTGATGTTAACCATG Diversispora G5fw2 GCCAGTGAAAATTCAGTTTGG aurantia G5rv2 TCAGTATCGGTTTCGGGAG C49fw GCGTGCGGCAGGGTAA C49rv CCTGCCATGCGGAATAGTG Diversispora D73fw TGGGTCGAGTCAGGGTCAA omaniana D73rv CGCTGACCTTCCAACAAAGAA Rhizophagus Rifw TCTGTGGAGTGTGAGGAGCTTAAC irregularis Rirv CAACCACACGGGCAAGTACA africanum Paraglomus sp. Amplicon size 127 126 71 79 76 Fig. 1 Standard curves for conversion of the cycle number (Cq) of the quantitative reverse transcription-PCR (qRTPCR) assay to numbers of rLSU copies per ng RNA for each of the five arbuscular mycorrhizal fungal (AMF) species (a) Septoglomus africanum, (b) Diversispora aurantia, (c) Paraglomus sp., (d) Diversispora omaniana and (e) Rhizophagus irregularis. For each of the three dilution series, the logarithm of the numbers of rLSU copies per ng RNA is plotted against the mean cycle number +SE required for the fluorescent signal to exceed the detection threshold. The linear regression lines and equations are shown.