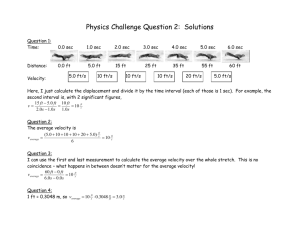
ch 21 problems

Name:________________
A) radionuclides
B) radioisotopes
____
21.1
1) Atoms containing radioactive nuclei are called __________.
C) nucleons
D) nuclides
E) radioisophores
2) In what type of radioactive decay does the atomic number of the product increase by one?
A) alpha
B) beta
C) gamma
D) positron emission
E) electron capture
3) In balancing the nuclear reaction
75
35
Br
→ E +
0
1 e, the identity of element E is
__________.
A) Kr
B) Br
C) U
D) Se
E) none of the above
4) What is the missing product from this reaction?
A)
4
2
He
B)
0
-1 e
32
15
P →
32
16
S + _____
C)
0
0
γ
D)
1
0 e
E)
1
0 p
Chapter 21 problems
5) Alpha decay produces a new nucleus whose __________ than those respectively of the original nucleus.
A) atomic number is 2 less and mass number is 2 less
B) atomic number is 1 less and mass number is 2 less
C) atomic number is 2 less and mass number is 4 less
D) atomic number is 2 more and mass number is 4 more
E) atomic number is 2 more and mass number is 2 less
22.2
6) At approximately what number of protons, or neutrons, does the 1:1 ratio of protons to neutrons start to produce unstable nuclei?
A) 10
B) 20
C) 30
D) 50
E) 80
7) What is the largest number of protons that can exist in a nucleus and still be stable?
A) 206
B) 50
C) 92
D) 83
E) 84
8) The largest number of stable nuclei have an __________ number of protons and an
__________ number of neutrons.
A) even, even
B) odd, odd
C) even, odd
D) odd, even
E) even, equal
9) The formation of krypton from rubidium decay is a result of __________.
A) alpha emission
B) beta emission
C) positron emission
D) electron capture
E) neutron capture
10) The largest number of stable nuclei have an __________ number of protons and an
__________ number of neutrons.
A) even, even
B) odd, odd
C) even, odd
D) odd, even
E) even, equal
21.3
11) What is required for a nuclear transmutation to occur?
A) very high temperature
B) a corrosive environment
C) a particle to collide with a nucleus
D) spontaneous nuclear decay
E) gamma emission
12) In the nuclear transmutation represented by
239
94
Pu(
4
2
He,
1
0 n)?, what is the product?
A) uranium-242
B) curium-245
C) curium-242
D) uranium-245
E) uranium-243
13) In the nuclear transmutation represented by
14
7
N(
1
0 n,
1
1 p)?, what is the emitted particle?
A) neutron
B) proton
C) positron
D) alpha particle
E) electron
14) The product of the nuclear reaction in which 28Si is subjected to neutron capture followed by alpha emission is __________.
A) 31S
B) 33S
C) 23Mg
D) 25Mg
E) 25Al
15) Cobalt-60 is produced by a three reaction process involving neutron capture, beta-emission, and neutron capture. The initial reactant in the production of cobalt-60 is __________.
A) 59Co
B) 56Fe
C) 58Fe
D) 61Co
E) 60Fe
21.4
16) The decay of a radionuclide with a halflife of 2.3 × 105 years has a rate constant (in yr-1) equal to __________.
A) 3.3 × 105
B) 3.0 × 10-6
C) 6.0 × 10-6
D) 2.8 × 103
E) 5.9 × 10-8
17) The beta decay of cesium-137 has a half-life of 30.0 years. How many years must pass to reduce a 25 mg sample of cesium 137 to 8.7 mg?
A) 46
B) 32
C) 3.2
D) 50
E) 52
18) The half-life of 218Po is 3.1 minutes.
How much of a 155 gram sample remains after 0.40 hours?
A) 0.00067 g
B) 0.0072 g
C) 0.72 g
D) 0.0047 g
E) none of the above
19) 210Pb has a half-life of 22.3 years and decays to produce 206Hg . If you start with
7.50 g of 210Pb, how many grams of 206Hg will you have after 17.5 years?
A) 4.35
B) 3.15
C) 3.09
D) 0.0600
E) 1.71
20) Cesium-137 undergoes beta decay and has a half-life of 30.0 years. How many beta particles are emitted by a 14.0-g sample of cesium-137 in three minutes?
A) 6.1 × 1013
B) 6.2 × 1022
C) 8.4 × 1015
D) 1.3 × 10-8
E) 8.1 × 1015
21) A rock contains 0.275 mg of lead-206 for each milligram of uranium-238. The half-life for the decay of uranium-238 to lead-206 is 4.5 × 109 yr. The rock was formed __________ yr ago.
A) 1.42 × 109
B) 9.62 × 108
C) 1.24 × 109
D) 1.79 × 109
E) 1.39 × 109
21.5+6
22) Which one of the following devices converts radioactive emissions to light for detection?
A) Geiger counter
B) photographic film
C) scintillation counter
D) none of the above
E) radiotracer
23) The mass of a proton is 1.673 × 10-24 g.
The mass of a neutron is 1.675 × 10-24 g.
The mass of the nucleus of an 56Fe atom is
9.289 × 10-23 g. What is the nuclear binding energy (in J) for a 56Fe nucleus? (c = 3.00 ×
108 m/s)
A) 2.57 × 10-16
B) 7.72 × 10-8
C) 8.36 × 10-9
D) 7.65 × 10-11
E) 6.07 × 106
24) When two atoms of 2H are fused to form one atom of 4He, the total energy evolved is 3.83 × 10-12 J. What is the total change in mass (in kg) for this reaction? (C
= 3.00 × 108 m/s)
A) 1.28 × 10-17
B) 4.26 × 10-26
C) 3.45 × 108
D) 1.15
E) 4.26 × 10-29
25) Carbon-11 decays by positron emission:
11
6
C →
11
5
B +
1
0 e
The decay occurs with a release of 2.87 ×
1011 J per mole of carbon-11. When 4.00 g of carbon-11 undergoes this radioactive decay, __________ g of mass is converted to energy.
A) 1.16 × 10-3
B) 3.48 × 105
C) 1.16 × 10-6
D) 8.62 × 102
E) 1.28 × 10-2
21.7
26) What is the typical percent of uranium-
235 in the enriched UO2 pellets used in nuclear reactors?
A) 0.7
B) 1
C) 3
D) 10
E) 14
27) What drives the turbine in a nuclear power plant?
A) the moderator
B) steam
C) the control rods
D) the primary coolant
E) UF6 gas
28) On average, __________ neutrons are produced by every fission of a uranium-235 nucleus.
A) 4
B) 3.5
C) 1
D) 2.4
E) 2
21.8
29) What type of reaction is known as a thermonuclear reaction?
A) fission
B) fusion
C) transmutation
D) beta emission
E) neutron emission
30) The main scientific difficulty in achieving a controlled fusion process is the
A) enormous repulsion between nuclei being fused.
B) enormous repulsion between the electrons of atoms being fused.
C) very large number of positrons emitted.
D) very large number of x-rays emitted.
E) very large number of gamma rays emitted.
21.9
31) Which one of the following forms of radiation can penetrate the deepest into body tissue?
A) alpha
B) beta
C) gamma
D) positron
E) proton
32) Which one of the following is not true concerning radon?
A) It decays by alpha emission.
B) It decays to polonium-218, an alpha emitter.
C) It is chemically active in human lungs.
D) It has been implicated in lung cancer.
E) It is generated as uranium decays.
33) When ionizing radiation enters the body, what is the predominant free radical produced?
A) H
B) H3O
C) protein
D) OH
E) H2O
Answers
1.
B Diff: 1 Sec. 21.1
2.
B Diff: 2 Sec. 21.1
3.
D Diff: 2 Sec. 21.1
4.
B Diff: 1 Sec. 21.1
5.
C Diff: 2 Sec. 21.1
6.
B Diff: 1 Sec. 21.2
7.
D Diff: 1 Sec. 21.2
8.
A Diff: 1 Sec. 21.2
9.
D Diff: 3 Sec. 21.2
10.
A Diff: 1 Sec. 21.2
11.
C Diff: 1 Sec. 21.3
12.
C Diff: 2 Sec. 21.3
13.
B Diff: 1 Sec. 21.3
14.
D Diff: 3 Sec. 21.3
15.
C Diff: 4 Sec. 21.3
16.
B Diff: 1 Sec. 21.4
17.
A Diff: 1 Sec. 21.4
18.
C Diff: 2 Sec. 21.4
19.
A Diff: 3 Sec. 21.4
20.
E Diff: 4 Sec. 21.4
21.
D Diff: 4 Sec. 21.4
22.
C Diff: 1 Sec. 21.5
23.
D Diff: 2 Sec. 21.6
24.
E Diff: 2 Sec. 21.6
25.
A Diff: 3 Sec. 21.6
26.
C Diff: 1 Sec. 21.7
27.
B Diff: 1 Sec. 21.7
28.
D Diff: 1 Sec. 21.7
29.
B Diff: 1 Sec. 21.8
30.
A Diff: 1 Sec. 21.8
31.
C Diff: 1 Sec. 21.9
32.
C Diff: 1 Sec. 21.9
33.
D Diff: 1 Sec. 21.9
Answers
1.
B Diff: 1 Sec. 21.1
2.
B Diff: 2 Sec. 21.1
3.
D Diff: 2 Sec. 21.1
4.
B Diff: 1 Sec. 21.1
5.
C Diff: 2 Sec. 21.1
6.
B Diff: 1 Sec. 21.2
7.
D Diff: 1 Sec. 21.2
8.
A Diff: 1 Sec. 21.2
9.
D Diff: 3 Sec. 21.2
10.
A Diff: 1 Sec. 21.2
11.
C Diff: 1 Sec. 21.3
12.
C Diff: 2 Sec. 21.3
13.
B Diff: 1 Sec. 21.3
14.
D Diff: 3 Sec. 21.3
15.
C Diff: 4 Sec. 21.3
16.
B Diff: 1 Sec. 21.4
17.
A Diff: 1 Sec. 21.4
18.
C Diff: 2 Sec. 21.4
19.
A Diff: 3 Sec. 21.4
20.
E Diff: 4 Sec. 21.4
21.
D Diff: 4 Sec. 21.4
22.
C Diff: 1 Sec. 21.5
23.
D Diff: 2 Sec. 21.6
24.
E Diff: 2 Sec. 21.6
25.
A Diff: 3 Sec. 21.6
26.
C Diff: 1 Sec. 21.7
27.
B Diff: 1 Sec. 21.7
28.
D Diff: 1 Sec. 21.7
29.
B Diff: 1 Sec. 21.8
30.
A Diff: 1 Sec. 21.8
31.
C Diff: 1 Sec. 21.9
32.
C Diff: 1 Sec. 21.9
33.
D Diff: 1 Sec. 21.9