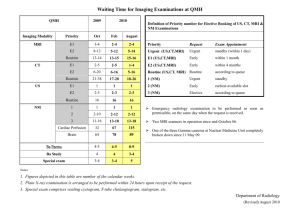
Detailed plan - Society for Pediatric Radiology
advertisement

Detailed plan and bibliography Purpose: 1. To determine the feasibility and acceptability of performing ‘FAST’ protocol non-anaesthetized / sedated MRI scans of the lungs in children with suspected pneumonia and to evaluate the quality of successful scans. 2. To interpret these scans for a diagnosis of pneumonia, associations that would point to a causative organism (e.g. fungus or tuberculosis) and detection of complications. Background Pneumonia is the leading infectious cause of morbidity and mortality in children < 5 years globally, accounting for 18% of deaths in this age group (1). Streptococcus pneumonia (the pneumococcus) and Haemophilus influenzae type b (Hib) are the most important causes of vaccine-preventable deaths in this age group (2, 3). In 2000, before the widespread use of bacterial conjugate vaccines, Streptococcus pneumonia caused an estimated 14.5 million cases of pneumococcal disease (95.6% pneumonia) and 826,000 deaths (2) while Hib caused 370 000 deaths (3). The UK had 50,844 episodes of lower respiratory tract infection from community acquired pneumonia in children aged 0-4 in 2010 (4). The world not only needs better rollout of preventative strategies and treatment, but also improved diagnostic techniques to make an aetiological diagnosis stronger. The introduction of the pneumococcal conjugate vaccine (PCV) demonstrated vaccine efficacy for radiograph confirmed pneumonia (5) of 20% in South Africa (HIV uninfected children) (6), 37% in Gambia (7), 18% in USA (8) and 23% (9) in the Philippines. New conjugate vaccines (PCV13) will not only change the aetiology (more staph + RSV) but also the severity of disease and occurrence of complications (e.g. initially there was an increase in empyaema reported after PCV7 was introduced and now there is a clear decline in empyaema with PCV13). Imaging may have a much larger role to play in diagnosis and management of complications of respiratory infection. TB is also a cause of acute pneumonia (8-15% high TB areas) and there is a higher reliance on imaging for making a diagnosis of TB than with community acquired pneumonias. Even though the British Thoracic Society advises against routine radiographs for children suspected of community acquired pneumonia (10), the chest X-ray remains the most readily available and the most common imaging modality for the assessment of childhood pneumonia (11). However, the inherent 2 dimensional limitations and inability to characterise lung infection according to aetiology have led to increased use of CT scanning of the chest. While CT may be the diagnostic solution in adults, it is undesirable in children because of the massive radiation dose to the developing organs, which has recently been highlighted as a major cause of iatrogenic increase in cancer rates in later life (12). In this context, the advantages of chest MRI are obvious – it does not involve ionizing radiation and provides high soft tissue contrast without a need for intravenous contrast agents. However, the length of standard MRI protocols (resulting in limited patient throughput) and the high cost are major stumbling blocks for expanding its use to diagnosing more common diseases affecting children. Recent technical advances in MR sequence speed, higher MRI signal, improved resolution and the increasing availability of scanners, demands reinvestigation of this modality in identification of more common pathologies. One of these is imaging of the lung for pneumonia as well as its associations and complications. Anaesthesia, which is currently used for MRI in young children because of the intimidating environment and for reducing patient motion encountered during the long procedure times, presents an unnecessary risk for patients with co-morbidities, including pulmonary and airway compromise as well as long-term neurodevelopmental risks, (particular for children younger than 2 or 3 years of age) (13). Anaesthesia also results in lung atelectasis, appearing as air-space disease in dependent areas that may require lung recruitment and controlled-ventilation technique for avoiding confusion with pathology (14). Despite these limitations, MRI has been used in children for diagnosing a range of thoracic diseases. Because of the excellent anatomic detail of the trachea and bronchi provided by MRI of the chest, it is particularly useful for evaluating suspected vascular rings and demonstrating aberrant vessels (15). Focal lesions such as abscess, tumour (15), lobar and segmental collapse (15, 16) and fibrosis are visible with MRI. Tumors such as lymphoma, neuroblastoma and pulmonary metastases demonstrate abnormal high signal intensity on T2 weighted imaging (15). MRI has also been evaluated as a potential imaging alternative in patients with cystic fibrosis (CF) (1618). MRI of the bone marrow allows accurate evaluation of the pathological conditions involving the bony thorax (15). New strategies are needed specifically for performing lung parenchymal MRI in young children for it to become a useful tool for diagnosing more common diseases with high morbidity, such as pneumonia. The principal investigator in the proposed study has previously reported abnormal high parenchymal signal abnormality from airspace disease on MRI as well as TBspecific low T2/STIR signal intensity in the parenchyma of children who underwent (unsedated /non-anaesthetised) MRI. The reason for the shortening of the T2 signal is not clear, but may be the result of the presence of paramagnetic free radicals in the enclosed macrophages in TB (19). Modified MRI may prove to be able to distinguish aetiological agents of pneumonia, something which chest radiographs are currently unable to do. In addition, lymphadenopathy (in diseases such as TB, fungal infection and lymphoma) can easily be detected and characterized to limit a differential diagnosis. In lymphoma lymphadenopathy demonstrates high signal on T2/STIR imaging, while TB lymph nodes may demonstrate characteristic low signal (19, 20). To improve access to MRI, Boiselle and colleagues recommend creating relationships to use MRI scanners outside of children’s hospitals in order to increase available MRI capacity (21). They also recommend use of multichannel coils, parallel imaging and new fast MRI sequences on 3T MRI scanners to improve image quality at decreased imaging times without sedation (21). We are further adding to these recommendations by determining the feasibility of limited sequences to develop nonreliance on sedation / anaesthesia which will cut costs and shorten MRI times. Limited sequences in the form of diffusion-weighted imaging (sensitive for pathology) and Short Tau Inversion Recovery (STIR) (characteristics of pathology) can be performed within 10-15 minute slots without sedation for children with suspected TB (unpublished research in progress Tanyia Pillay, Savvas Andronikou, Heather Zar - University of Cape Town). A successful feasibility study of ‘FAST’ MRI for common lung diseases in British children can serve to transform MRI into a more routine modality for diagnosis of common diseases affecting children, such as pneumonia and its complications. Plan of investigation A prospective feasibility study is proposed for imaging children with suspected pneumonia using ‘FAST’ limited sequence MRI specifically aimed at imaging the lung parenchyma. Children 13 years of age and younger will be enrolled at Bristol Royal Hospital for children when they are referred for chest X-ray for suspected pneumonia. Once the radiograph has been taken and they have initiated therapy the research assistant in the study will meet / contact the parents to explain the study and request the child’s participation and consent. Possible participants names will be obtained from the PACS [digital imaging archive] in the department of radiology during the reporting of radiographs based, on the clinical request and not on the chest X-ray findings. Patients will be excluded if the radiograph was not performed for any reason and if the patients / parents /carers do not consent to participation. Consecutive patients will be selected until 100 have been recruited. Patients will also be excluded if they are admitted in ICU or if they are in regular wards and are too ill to be moved to the MRI suite. Recruitment is therefore expected to reflect communityacquired pneumonia in ambulant patients in the majority. MRI scans will be performed at CRICBristol (a research MRI facility based within a Hospital setting) or BRHC which both offer high field strength 3 Tesla Siemens Magnetom Skyra which has a large bore and short length especially designed to increase patient comfort and compliance for faster MRI sequences and allows for 20 minute scanning slots at a reduced cost. The MRI protocol will include axial DWI B500, which will be post-processed in the coronal plane and inverted for viewing, and coronal STIR imaging. These sequences provide the sensitivity and specificity for detecting air-space disease, lymphadenopathy and effusions respectively. Patient safety: MRI will not be performed during the acute presentation but only after the patient has stabilized. It is well known that the radiological resolution of pneumonia lags significantly behind the clinical improvement and that air-space disease remains positive for some time, allowing for an adequate window to perform a scheduled MRI that will not endanger the patient. MRI scans will therefore be scheduled within 3 days of the initial presentation for CXR. No sedation or anaesthesia will be used. No intravenous contrast will be administered. MRI carries no risk of ionising radiation. Care will be taken as is standard procedure in MRI units to avoid the inherent risks of the high magnetic field e.g. metallic objects shall be removed from pockets etc. Image interpretation: Images will be reviewed by 3 pediatric radiologists blinded to the clinical findings, CXR findings and each other. The majority consensus will be recorded. Parameters recorded on a standardised recording card will include presence, location, extent and signal of air-space disease, lymphadenopathy, effusion and any other intra-and extrathoracic abnormality noted. Chest radiographs will also be reviewed at a separate sitting (2 months after the MRI reading) by the 3 radiologists blinded to each other. The MRI findings and clinical information and the findings will be recorded on a similar standardized recording card as for MRI. The consensus findings for the two modalities will be compared and the MRI findings will be compared against the final laboratory diagnosis where possible to determine whether MRI was able to predict the aetiological agent. Feasibility of ‘FAST’ MRI for diagnosis of community acquired pneumonia will be reported as the ‘number of completed scans’, which will be graded for quality (do we have a scale and criteria for scan quality evaluation?adequate clinical quality; poor quality but interpretatble; poor quality and uniterpretable). Reasons for support requested The financial support is required for funding the MRI scans for each child enrolled in the study. The MRI scanners are available either on-site at the Bristol Royal Hospital for Children or off-site at CRICBristol which is situated within St. Michaels Hospital nearby. References: 1. Black R E, Cousens S, Johnson H L, Lawn J E, Rudan I, Bassani D G, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969-1987. 2. O'Brien K L, Wolfson L J, Watt J P, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893-902. 3. Watt J P, Wolfson L J, O'Brien K L, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903-911. 4. Rudan I, O'Brien K L, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. Journal of global health. 2013;3:010401. 5. Cherian T, Mulholland E K, Carlin J B, Ostensen H, Amin R, de Campo M, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bulletin of the World Health Organization. 2005;83:353-359. 6. Madhi S A, Kuwanda L, Cutland C, Klugman K P. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIVinfected and -uninfected children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;40:1511-1518. 7. Cutts F T, Zaman S M, Enwere G, Jaffar S, Levine O S, Okoko J B, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebocontrolled trial. Lancet. 2005;365:1139-1146. 8. Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen J R, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. The Pediatric infectious disease journal. 2000;19:187-195. 9. Lucero M G, Nohynek H, Williams G, Tallo V, Simoes E A, Lupisan S, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. The Pediatric infectious disease journal. 2009;28:455-462. 10. Bowen S J, Thomson A H. British Thoracic Society Paediatric Pneumonia Audit: a review of 3 years of data. Thorax. 2013;68:682-683. 11. Pitcher R D, Lombard C, Cotton M F, Beningfield S J, Zar H J. Clinical and immunological correlates of chest X-ray abnormalities in HIV-infected South African children with limited access to anti-retroviral therapy. Pediatric pulmonology. 2014;49:581-588. 12. Mathews J D, Forsythe A V, Brady Z, Butler M W, Goergen S K, Byrnes G B, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. 13. Cauldwell C. Anesthesia risks associated with pediatric imaging. Pediatric radiology. 2011;41:949-950. 14. Newman B, Krane E J, Gawande R, Holmes T H, Robinson T E. Chest CT in children: anesthesia and atelectasis. Pediatric radiology. 2014;44:164-172. 15. Kangarloo H. Chest MRI in children. Radiologic clinics of North America. 1988;26:263-275. 16. Failo R, Wielopolski P A, Tiddens H A, Hop W C, Mucelli R P, Lequin M H. Lung morphology assessment using MRI: a robust ultra-short TR/TE 2D steady state free precession sequence used in cystic fibrosis patients. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;61:299-306. 17. Puderbach M, Eichinger M, Gahr J, Ley S, Tuengerthal S, Schmahl A, et al. Proton MRI appearance of cystic fibrosis: comparison to CT. European radiology. 2007;17:716-724. 18. Puderbach M, Eichinger M, Haeselbarth J, Ley S, Kopp-Schneider A, Tuengerthal S, et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: comparison to thin-section CT and chest x-ray. Investigative radiology. 2007;42:715-725. 19. Peprah K O, Andronikou S, Goussard P. Characteristic magnetic resonance imaging low T2 signal intensity of necrotic lung parenchyma in children with pulmonary tuberculosis. Journal of thoracic imaging. 2012;27:171-174. 20. Andronikou S, Vanhoenacker F M, De Backer A I. Advances in imaging chest tuberculosis: blurring of differences between children and adults. Clinics in chest medicine. 2009;30:717-744, viii. 21. Boiselle P M, Biederer J, Gefter W B, Lee E Y. Expert opinion: why is MRI still an under-utilized modality for evaluating thoracic disorders? Journal of thoracic imaging. 2013;28:137.