lab5final
advertisement

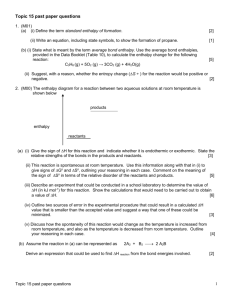
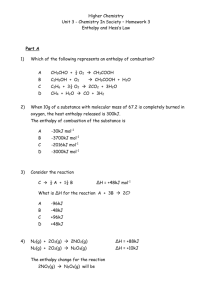
Lab # 5 Experimental Application of Hess's Law Experiment 2: The heat of formation of solid Calcium Hydroxide Diana Bakos Stein van der Plas Liam Beck SCH4U 01 November, 2014 Introduction The first law of thermodynamics states that during a chemical reaction energy is conserved. Hess's law follows this application because if a reaction were to proceed in a series of steps the enthalpy change for the overall reaction would equal the sum of all the enthalpies for the individual steps. This is due to the fact that enthalpy is a state function it does not does not depend on the pathway taken to convert reactants into products. Furthermore, Hess's law states that one can add up two or more separate equations to arrive at a target chemical equation. This can also help determine the enthalpy of the target equation because all the separate enthalpies of the individual reactions would equal that of the target equation. This is the reason as to why chemists view Hess's law as one of the most important concepts in chemistry. It can be used to find the enthalpy values for reactions that may be difficult to perform directly or too dangerous perform directly. Therefore, Hess's law can be used to determine the overall energy required for a chemical reaction, without even carrying out said experiment. In the following experiment, applications of Hess's law were used to determine the change of enthalpy for the formation of solid Calcium Hydroxide Ca(s) + O2(g) + H2(g) → Ca(OH) by adding two separate equations. A coffee cup calorimeter was used to calculate the enthalpy of the first reactions. The first reaction conducted was that of Ca(s) + 2 H2O(l) -----> Ca(OH)2(s) + H2(g) . The enthalpy of the other reaction needed H2(g) + ½O2(g) → H2O(l) was researched. Objectives Please refer to page 1of 2 Materials Please refer to page 1of 2 Procedure Please refer to page 2of 3 Observations: Temperature Measurements for the Enthalpy of Combustion of Magnesium ribbon Time (seconds) Experiment 2: Equation 5 Ca(s) + 2 H2O(l) -----> Ca(OH)2(s) + H2(g) 0s 22.5 oC 30 40.3 oC 60 43.1 oC 90 43.0 oC 120 42.8 oC 150 42.6 oC 180 42.4 oC 210 42.2 oC 240 42.1 oC 270 300 41.9 oC 41.8 oC Temperature of Ca(s) + 2 H2O(l) 43.5 43 Temperature (deg C) 42.5 42 41.5 Temperature 41 40.5 40 30 60 90 120 150 180 210 240 270 Time (s) Data Analysis- Experiment 2: Part 3: Equation 7: Ca(s) + 2 H2O(l) -----> Ca(OH)2(s) + H2(g) Data Collected Volume of 1.0 M HCl solution used Mass of magnesium 100mL 0.099g 300 Initial temperature of H2O solution before mixing Final temperature of Ca(OH)2(s) solution Calculations 22.5 oC ΔT =Tfinal -Tinitial =42.4 oC -22.5 oC =20.9oC Q= mc ΔT Energy produced in reaction 42.8 oC = (100g)(4.184J/g oC) (20.3oC) =-8494J Moles of Calcium used ΔH 0.99gCa× = = 1𝑚𝑜𝑙 𝐶𝑎 40.08𝑔𝐶𝑎 = 0.025molCa qrxn n 8494J 0.025mol = -340kJ/mol-1 eqn (6) 2H2(g) + O2(g) -------> 2H2O(l) ΔH2= -572 kJ/mol-1 ×2 eqn (7) Ca(s) + 2 H2O(l) -----> Ca(OH)2(s) + H2(g) ΔH2 = 3.4x102kJ/mol-1 ______________________________________________________________________________ eqn (5) Ca(s) + O2(g) + H2(g) ----> Ca(OH)2(s) ΔH5 = ΔH6 + ΔH7 = 2(-572 kJ/mol-1) + (-3.4x102kJ/mol-1) = -1484 kJ/mol-1 % 𝑒𝑟𝑟𝑜𝑟 = (−804kJ · mol − 1) − (−987 kJ · mol − 1) (−987 kJ · mol − 1) % error = 18.54% Discussion Through the following experiment the molar enthalpy of the formation of calcium hydroxide was calculated to be -804 kJ/mol-1. The following result was calculated by using a coffee cup calorimeter to determine the enthalpy of Ca(s) + 2 H2O(l) → Ca(OH)2(s) + H2(g), which was +3.4x102kJ/mol-1. The enthalpy of the last reaction needed was researched. According to Chemwiki H2(g) + ½O2(g) → H2O(l) has the molar enthalpy of -572 kJ/mol-1. In order to calculate the enthalpy change of the equation for the formation of calcium hydroxide the separate enthalpies were manipulated using Hess's law. It was observed that the reaction was exothermic because the final temperature was greater than the initial temperature. However, the following experiment was not accurate because the value calculated holds a 18.54% error compared to the theoretical value. The error could have been a result of heat being lost to the surroundings instead of being absorbed into the system, the assumptions made in the calculations and faulty time readings. Heat was lost to the surrounding because the coffee-cup calorimeter was a closed system. To maximize the efficiency of the calorimeter within the lab one could include an insulator. Preferably one, that could decelerate the effect of heat flow by conduction of convection. Another, source of error was the assumptions needed to be made to complete the lab calculations. The experiment called for one to assume that the solution had the same specific heat capacity as water. This error cannot truly be improved, but one could conduct the experiment several times to calculate several heats of formation of calcium hydroxide and then arrive at an average. This would probably help with the accuracy of the experiment. The last source of error that will be discussed is faulty time reading. During the experiment, there may have been times when the reactants did not fully mix, which meant that the reaction did not reach its full potential. Hence, the wrong temperature could have been recorded during the different time intervals. To insure that that the solution fully reacted the experiment could be recorded over a longer time interval. Source of error The errors that occurred in this lab could have been a result of heat being lost, the assumptions made in the calculations and faulty time readings. Due to the coffee-cup calorimeter being a closed system, heat was exchanged between the system and the surroundings. Consequently, the temperature of the air surrounding the system increased. Since, heat is always transferred from an object at higher temperature to the object at lower temperature until thermal equilibrium is reached the warm air, which is less dense than cold air, was forcibly replaced by the cold air. This process turned into a continues cycle. To maximize the efficiency of the calorimeter within the lab one could include an insulator. Preferably one, that could decelerate the effect of heat flow by conduction of convection. For example, cotton is an excellent insulator because the thousands of tiny air spaces between the fibers of the cotton would slow down the rate of transmission of energy by trapping it. Another, source of error was the assumptions needed to be made to complete the lab calculations. The experiment called for one to assume that the solution had the same specific heat capacity as water. Chemists usually assume that the specific heat capacity of a dilute solution is very close to the specific heat capacity of pure water. Hence, the specific heat capacity of HCl may not be 4.184 J K-1 g-1, but is somewhat precise to what the true value may be. This error cannot truly be improved, but one could conduct the experiment several times to calculate several heats of combustion for magnesium and then arrive at an average. This would probably help with the accuracy of the experiment. The last source of error that will be discussed is faulty time reading. During the experiment, there may have been times when the reactants did not fully mix, which meant that the reaction did not reach its full potential. Hence, when the waiting for the solution to reach its maximum level, the thermometer may have read that the temperature was cooling or at a constant and that time could have been recorded. To improve this error, the experiment could be recorded over a longer time interval because that would insure that the solution had fully reacted. The solution may not have fully reacted at the beginning. Conclusion In conclusion, the molar enthalpy of the combustion of solid Calcium Hydroxide was -1484 kJ/mol-1The following result was calculated by using a coffee cup calorimeter to determine the enthalpy of Ca(s) + 2 H2O(l) → Ca(OH)2(s) + H2(g), which was +3.4x102kJ/mol-1. The enthalpy of the last reaction needed was researched. According to Chemwiki H2(g) + ½O2(g) → H2O(l) has the molar enthalpy of -572 kJ/mol-1. A series of steps were followed to do the calculation, which applied Hess's law. This law states that enthalpy is a state function and that it is possible to arrive at a target equation ( Ca(s) + O2(g) + H2(g) ----> Ca(OH)2(s)) by adding up two or more separate equations because the sum of all the enthalpies for the individual steps would equal the overall reaction. Overall, the reaction only had a 18.54% error, which suggests that the experiment was inaccurate. Work Cited