what is a mineral and how does it form worksheet
advertisement

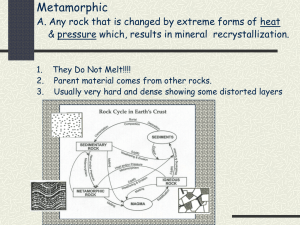
What is a mineral and how does it form? Definition of a Mineral Minerals are the fundamental building blocks of rock and, as such, form the basis for our understanding of the science of the Earth. In fact, the study of minerals (mineralogy) makes up one of the two original subdivisions of Geology, the other being the study of rock layers – stratigraphy. Before we formulate a detailed description of the common minerals and their properties, it is first necessary to define exactly what is meant by the term mineral. Minerals are naturally occurring, solid substances, of inorganic origin (never alive), with specific (but not necessarily fixed) chemical compositions and a crystalline structure. Examining this multi-part definition sequentially will allow us to understand the role of minerals in geology more clearly. First, minerals are naturally occurring. Thus, the products of industrial or commercial processes are not considered true minerals, no matter how well they fit the rest of the definition. Industrially produced minerals such as synthetic rubys or sapphires are readily recognized because of their purity of chemical composition. Nature is considerably more messy than a laboratory. Second, minerals are solid substances with a crystalline structure. To be crystalline means that the atoms within the solid are arranged in a geometric pattern that is unique to that mineral. Thus, altering that structure in any way results in the creation of a new mineral. Additionally, substances formed from the other phases of matter, liquid and gas, are not minerals. For that reason, water ice from a glacier is a mineral, while liquid water, steam, and ice cubes are not. Third, minerals are of inorganic origin. That is to say, they are not organic compounds such as amino acids, peptides, or enzymes. Interestingly, however, many organic processes result in the formation of minerals. For instance, seashells are made of minerals, as are coral reefs and the bones and teeth of animals. An easy rule of thumb is that if a material is associated with the "soft-parts" of a plant or animal it can not be a mineral while if that material is part of the skeleton or rigid support structure then it might be composed of minerals. Finally, minerals have a chemical composition. This means that a mineral has specific types of atoms in its structure that occur in the same amount each time it forms. It is, therefore, possible to write a chemical formula for any mineral. Some minerals have simple chemical formulas, such as the mineral halite (table salt) we encountered earlier – NaCl. Others have significantly more complex chemical compositions, such as the common rock forming mineral mica KAl3Si3O10(OH)2. Importantly, the chemical composition of a mineral is an integral part of its definition. However, some chemical variation is allowed. Natural impurities are common in minerals, as is the tendency for one element to slip into the crystalline structure in place of some other element. Substitutions of these types do not cause a change in mineral name as long as the replacements make up only a small percentage of the total structure. Chemical variation most commonly is associated with the replacement of cations (atoms with a positive charge, like a magnet) within the mineral. Thus, table salt usually contains a few percent potassium (K) replacing the sodium (Na) at the cation positions within the structure. Substitutions and other forms of impurities are of great interests to geologists because they provide clues to the nature of the processes responsible for the formation of the mineral. Atomic Structure of Minerals Minerals are crystalline solid substances, meaning the atoms making up a mineral are arranged in an ordered, three-dimensional, structure. The distances and angles between an individual atom and the neighbors it is bonded to are constant. The result of this is that the structure of a crystal is both geometrical and highly symmetrical, forming a pattern unique to each mineral. The question remains, however, how do minerals form? How is this perfect geometric arrangement achieved within the complex and often chaotic natural world? The process of mineral formation is known as crystallization. In order for a mineral to crystallize, ions from the nearby environment must be brought together. There exist three primary mechanisms for mineral crystallization. The process most familiar to our everyday lives is the precipitation of minerals from a saline solution. Salty water, such as seawater, contains electrically charged atoms or groups of atoms known as ions. When seawater evaporates, the water becomes saltier. This is due to the concentration of ions in the water remaining after evaporation. As more and more evaporation occurs, the saltiness of the water increases. Eventually the water reaches a salinity where the concentrated ions begin to link together to form mineral crystals. While seawater provides a good example because of its high initial salinity, any natural water will precipitate some minerals if evaporation occurs long enough. For instance, think of the Great Salt Lake in Utah and the Dead Sea in the Middle East. Both of these lakes receive fresh river water that has a relatively low concentration of ions. However, due to the arid regional climate, the water in these lakes evaporates at a very rapid rate, continually increasing the salinity until minerals are formed. A second process of mineral formation occurs during cooling of a melt. When crystallization of this type takes place in water, we call it freezing. Through a very similar mechanism, molten rock-forming liquids, known as magmas and lavas, cool and crystallize to form minerals and thus rocks. Most rocks that occur in the crust of the Earth have formed by cooling from a melt and, as such, crystallization during cooling is the most important process of mineral formation on Earth. The third, and final, process of mineral formation is harder to conceptualize than either of the previously discussed mechanisms. Any particular mineral is stable over a range of temperatures and pressures – known as its stability field. When the pressure or temperature changes, the mineral may become chemically unstable. Crystallographic transformations (mineral/atom rearrangement) can then occur in the solid state to produce a mineral that is in equilibrium (more stable) with the new environment. Likewise, when various minerals are in close contact and temperature or pressure changes, especially in the presence of a fluid like water, chemical reactions occur to cause the dissolution (dissolving) of old minerals and creation of new ones. This process is of particular importance in tectonically dynamic regions of the Earth, where temperatures and pressures experienced by minerals change at relatively fast rates. The crystalline arrangement of atoms within a structure is a critical part of the definition of a mineral. Interestingly, some minerals have identical chemical compositions but different geometric arrangements of their constituent atoms and are known as polymorphs. These pairs, or groups, of minerals sharing identical chemistry but different structure are common and important to our understanding of processes of mineral formation. The most widely known polymorphic pair is formed from carbon – diamond and graphite. While both minerals are composed exclusively of carbon atoms, their physical characteristics could not be more different, thereby illustrating the importance of crystal structure to mineralogy. Name: _________________________________ Period:______________ 1. What four or five things make a mineral a mineral? (define it) 2. Are minerals naturally occurring? What does this mean? 3. What does it mean when a mineral has a crystalline structure? 4. Can you write a chemical formula for a mineral? What does this mean? 5. What does it mean when a mineral has a natural impurity? Give an example. 6. What is crystallization? How does it occur? 7. Describe the process of crystallization as it occurs when water evaporates: 8. How do minerals form when a ‘melt’ (molten/liquid rock) cools? 9. In the last process of mineral formation, how can minerals form/change through heat & pressure? 10. What happens to minerals under heat and pressure when they come into contact with water?