the chemistry of natural waters taken from a kutztown creek, a
advertisement

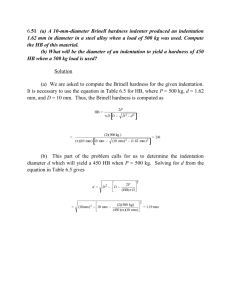
THE CHEMISTRY OF NATURAL WATERS TAKEN FROM A KUTZTOWN CREEK, A JAKARTA TAP, A PITTSBURGH TAP, AND A STATE COLLEGE TAP Experiment 10 NOVEMBER 12, 2013 ALEXANDRIA CONTI Chem 111 Section 104 Group Members: Cory Colligan, Meaghan Coleman, Chris Collier TA: Ray Regan Introduction: Leonardo da Vinci was the epitome of the term “Renaissance Man” in the late 1400’s. He was a brilliant painter, architect, anatomist, geologist, inventor, and engineer. In his time, he created such masterful artworks that even today are being sold for millions of dollars. Such an established historical figure has had a great influence on the world today, and the things that he has said have been written down and preserved for generations. One such quote that applies to this lab is “Water is the defining source in nature (1).” Indeed, water is needed for almost every aspect of nature. Without water, there would be no life. Humans, in fact, are up to 60% water (2). Water in the human body can be found in the brain, heart, lungs, muscles, kidneys, bones, and skin and it is used as a building material, an internal body temperature regulator, a transporter of vital carbohydrates and proteins, to flush out our systems through urination, and a shock absorber for the brain, spinal cord, and (in some cases) fetus (2). Because water is so vital to life, it is important that humans take in a sufficient amount each day from a clean source to prevent disease and illness. Most of the water utilized for ingestion in the modern world is from ground water sources. These sources have held water for over hundreds of years, and this aging poses a certain problem. As water trickles and settles underground, it dissolves small amounts of naturallyoccurring minerals which contributes to the hardness of the water supply (3). Because water is such a great solvent, these minerals are picked up quite easily. Hard water is water that is high in dissolved minerals, particularly calcium and magnesium (3). If hard water is not properly treated, it can result in many household problems (3). Health-wise, hard water poses very little risk (4). However, it causes many household problems. Hard water interferes with almost every cleaning task (4). It can make clothes feel rough, it causes glasses to get spotted, and it results in a film that can build up on shower doors, bathtubs, sinks, and faucets (4). What is considered the worst problem of all, though, is when minerals build up on plumbing fixtures (4). This buildup can clog pipes that results in reduced water flow and ultimately results in pipe replacement (4). So, it is preferred that water is only slightly hard, and we measure water hardness in many ways. The two water hardness measurements dealt with in this lab are EDTA Titration and Atomic Absorption (AA) Spectrophotometry. EDTA Titration works in steps (5). First, a known volume of water is taken and the pH is adjusted to 10 through the addition of a NH3/NH4 buffer (5). Next, EBT indicator is added to the solution (5). The indicator then reacts with the Mg2+ in the sample, forming a red color (5). EDTA solution is added to the solution next (5). This solution then reacts with the Ca2+ to form a colorless chelate first, then it reacts with the Mg2+ to produce a colorless MgEDTA chelate (5). The endpoint of the titration occurs when the Mg2+ is removed from the indicator and the indicator returns to its blue color (5). The endpoint of the titration is then used to determine the hardness of the water sample through M1V1 = M2V2. AA Spectrophotometry uses light to determine the hardness of water by measuring the amount of Ca2+ and Mg2+ (5). Monochromatic light is projected through the sample (5). The atoms in the sample with electronic energy separation matching the ∆E of the light will absorb the light (5). The absorbance is proportional to the concentration of the metal atoms in the sample, allowing us to use Beer-Lambert law to calculate the unknown metal concentration in the sample (5). The ∆Es used in our AA Spectrophotometry correspond to Ca2+ and Mg2+, allowing us to determine the hardness of water by determining the amount of the ions of the most abundance. We use two different techniques in this lab for two reasons. First, it allows us to determine the precision of our sample. Ideally, we should get the same value for both EDTA and AA analysis. However, EDTA titration measures all 2+ ions found in the sample while AA analysis only measures the amount of Ca2+ and Mg2+ (5). So, it is expected that the EDTA determination of hardness will be a tad bit higher than the AA analysis, because there are smaller amounts of other ions dissolved in water because water is such a great solvent. Because EDTA measures these ions and AA does not, the EDTA determination should be higher, though not significantly. Typically, hardness of water is reported in ppm as CaCO3 (6). This measurement is used because it is most convenient. Hardness values are very small, so ppm must be used in order to keep them useable in calculations (6). CaCO3 is used because it assumes that carbonate and calcium are the main contributors to hardness (6). This makes it easy to determine and compare hardness when, in reality, there are countless 2+ ions involved. Water is considered soft if its concentration in ppm CaCO3 is less than 75 (6). If the concentration is between 75 and 150, the sample is considered moderately hard (6). If the concentration is between 150 and 300, the sample is considered hard (6). If the sample has a concentration above 300, then it is considered very hard (6). In order to keep the hardness of water at an acceptable level, water softening techniques are used. All these techniques work in basically the same way: by removing Ca2+ and Mg2+ ions from water and replacing them with sodium ions. Sodium ions are known for their non- interference in household soaps and detergents and for the fact that they do not build up in pipes, which is perhaps the greatest and most helpful result. In our lab, we tested water from three taps (one from State College, Pa, one from Jakarta, Indonesia, and one from Pittsburgh, Pa) and one creek (from Kutztown, Pa). It is predicted that the creek will provide the hardest water sample, then the tap waters will follow behind it. The hardness levels of each tap sample will depend on the deposits of rock found near the groundwater sources and the level of treatment provided to each source. The Kutztown creek should end up being the hardest due to its location and general makeup (7). Given that this sample came from a creek not meant for water consumption, the water is not regulated or treated by any agency. Because of this, the large amount of ions it has accumulated over time from the environment have not been filtered out. These ions are what directly contributes to hardness, so it’s natural that the water will end up being harder. All of the other samples in this lab come from different taps, meaning they have been treated, unlike the creek. Also, the creek is located in Kutztown, which has a very interesting geological map (7). There are large deposits of limestone found near the location of the creek (7). Limestone contains a large mixture of calcium and magnesium, the two ions primarily responsible for water hardness (8). Therefore, the limestone deposits and the fact that the creek is unfiltered is what contributes to the high level of hardness in this sample. Some tap water sources are “groundwater” samples. This means that they are pumped from wells of naturally occurring water found 100s of feet in the ground (9). A perforated cone draws in groundwater and pumps it to the surface (9). Each groundwater source has been evolving for hundreds of years. This means that the rock formations found near the source will have had an effect on the hardness of the water, as ions (particularly calcium and magnesium ions) dissolve into each source (9). So, each well source will contain a certain level of hardness (9). It is up each county to make their water sources safe for consumption. The State College Borough Water Authority promises proper filtration of their water sources in order to have the hardness of their samples to fall within a certain range (10). Given the location of State College on a map of the United States of America, we are able to determine that the water found here is known to be moderately-hard to hard (3 to 10 gpg) (11). This means that, although the water is clean, it needs an extra amount of filtering to ensure that it meets the quality guidelines the Borough advertises on its website. Because the water is typically harder than average, it would fall on the upper end of the allowed range of hardness. Also, just like the Kutztown creek, State College is found between large deposits of limestone (10). These stones, once again, contribute greatly to the water hardness of the sample. The water from Pittsburgh, when determining its location of the United States Hardness Map, is extremely hard (14+ gpg) (11). This means that the sample from Pittsburgh should be much harder than the water from State College. However, given that that the Pittsburgh Water and Sewer Authority takes extra care when filtering its source because it is much dirtier, the Pittsburgh sample should be less hard than the Kutztown creek and State College samples. The PWSA draws its water from the Allegheny River, rather than the ground as State College does (12). Ground water is known to be much cleaner than river water, so the PWSA must take extra steps when filtering its water for consumption (12). In the end, after three rounds of clarification, filtration, and disinfection, the drinking water of Pittsburgh is certainly clean and much less hard than that of State College, due to the amount of filtration that occurs in order to remove harmful chemicals and microorganisms (12). The water from Jakarta, Indonesia is especially polluted (13). So, the city water authority (aka PAM) must take extra steps when making the water safe to use. PAM runs water from a water line directly into a holding tank on each home’s property (14). This makes it up to the home to ensure the quality and safety of their water (14). So, the hardness of water for each family depends on their commitment to having clean drinking water. Because it is in the hands of the individual, this sample is expected to be one of the softest (either harder or softer than the Pittsburgh tap sample). Procedure: (Reference PSU Chemtrek (5) for complete step-by-step instructions) Section A: Determination of Water Hardness by Atomic Absorption Spectroscopy (AA) - The AA method described above was used to determine the hardness of your water sample due to Ca2+ and Mg2+. Graphs were calibrated that allowed for the calculation of total hardness value Section B: Evaporation of Your Water Sample to Give Total Dissolved Solids - One drop of the water sample, one drop of distilled water, and one drop of a 1.0 × 10−4 𝑀 Ca2+ standard solution were evaporated on a piece of tin foil using a hot plate in order to determine total dissolved solids (TDS). Section C: Divalent Cation Analysis by EDTA Titration - EDTA titration as described in the introduction was completed on a 1.0 × 10−3 𝑀 sample of Ca2+ solution and a sample of a 1.0 × 10−3 𝑀 Mg2+ solution in order to practice the method of serial dilution and the use of the equation M1V1 = M2V2, which is used in determining water hardness. Section D: Determination of the Hardness of Your Water Sample - EDTA titration as described in the introduction was completed on the water sample. Next, the formula M1V1 = M2V2 was used in order to determine the hardness of the water sample, using drop number for volume and concentration of 2.00 × 10−4 𝑀 EDTA was used for known concentration. Section E: Water Softening with a Commercial Water-Condition Agent - A commercial water-conditioning agent was added to the water sample in order to soften it. EDTA titration was used in the same way as above in order to determine the new hardness of the sample. Section F: Divalent Cation Removal by Ion Exchange - A cation exchange resin was added to the water sample in order to soften it. Once again, EDTA titration was utilized to determine the new hardness of the sample. Results: Tables associated with water hardness: Table 1: Absorbance values of Ca and Mg through AA Analysis Water Sample Dilution Ca Absorbance Mg Absorbance PSU (15) 1:1 0.2459 0.1999 Pittsburgh (16) 1:1 0.1980 0.0961 Jakarta (17) 1:1 0.0539 0.0281 Kutztown (18) None 0.4001 0.3851 Table 2: Number of drops needed for EDTA Titration Water Sample Dilution Unsoftened Sample Baking Soda Softened Resin Softened PSU (15) 1:1 7 6 1 Pittsburgh (16) 1:1 4 3 1 Jakarta (17) 1:1 4 2 1 Kutztown (18) None 14 13 1 Table 3: Water hardness of each sample through EDTA Titration Water Sample Dilution Unsoftened Sample Baking Soda Softened Resin Softened PSU (15) 1:1 1.4 × 10−3 𝑀 1.2 × 10−3 𝑀 2.0 × 10−4 𝑀 Pittsburgh (16) 1:1 8.0 × 10−4 𝑀 6.0 × 10−4 𝑀 2.0 × 10−4 𝑀 Jakarta (17) 1:1 8.0 × 10−4 𝑀 4.0 × 10−4 𝑀 2.0 × 10−4 𝑀 Kutztown (18) None 2.8 × 10−3 𝑀 2.6 × 10−3 𝑀 2.0 × 10−4 𝑀 𝑀1 𝑉1 = 𝑀2 𝑉2 Where M= molarity of substance and V= volume (in drops) Sample calculation to find water hardness of Pittsburgh unsoftened sample: (2.00 𝑥 10−4 )(4 𝑑𝑟𝑜𝑝𝑠) = 𝑀2 (1 𝑑𝑟𝑜𝑝) 𝑀2 = 8.0 𝑥 10−4 𝑀 (These calculations use drop number from Table 2) Tables associated with Divalent Cation Removal and TDS residue comparison: Table 4: pH of water samples before and after Divalent Cation Removal by Ion Exchange Water Sample pH Before pH After PSU (15) 6 4 Pittsburgh (16) 6 4 Jakarta (17) 9 3 Kutztown (18) 8 4 Table 5: Comparison of TDS residue after evaporation of a sample Water Sample Observation Distilled Water Faintest residue – almost invisible 1 × 10−3 𝑀 Ca2+ Faint white ring PSU (15) Heavy white ring compared to reference Pittsburgh (16) Heavy white ring compared to reference Jakarta (17) Heavy white ring compared to reference Kutztown (18) Heavy white ring compared to reference Calibrated graphs allowing for the calculation of total hardness through AA analysis: Light Absorbance vs. Calcium Ion Concentration Light Absorbance Value (at 422.7 nm) 0.5 y = 0.009x + 0.0122 0.45 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 0 10 20 30 40 50 60 Calcium Ion Concentration (ppm) Figure 1: Calibration curve for Ca2+ concentration by AA analysis (at 422.7 nm) Light Absorbance vs. Magnesium Ion Concentration Light Absorbance Value (at 202.5 nm) 0.45 0.4 y = 0.0128x + 0.0106 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 0 5 10 15 20 25 30 35 Magnesium Ion Concentration (ppm) Figure 2: Calibration curve for Mg2+ concentration by AA analysis (at 202.5 nm) Determination of total hardness through AA analysis: Ca: 0.1980 0.1980 = 0.009𝑥 + 0.0122 X = 20.6 100 𝑔 𝐶𝑎𝐶𝑂3 20.6 × ( 1 𝑚𝑜𝑙𝑒 ) = 51.5 𝑝𝑝𝑚 𝐶𝑎𝐶𝑂3 = 𝟓𝟏. 𝟓 𝒑𝒑𝒎 𝒉𝒂𝒓𝒅𝒏𝒆𝒔𝒔 40.0 𝑔 𝐶𝑎2 + 1 𝑚𝑜𝑙𝑒 Mg: 0.0961 0.0961 = 0.0128𝑥 + 0.0106 X = 6.68 100 𝑔 𝐶𝑎𝐶𝑂3 1 𝑚𝑜𝑙𝑒 6.68 × ( ) = 27.5 𝑝𝑝𝑚 𝐶𝑎𝐶𝑂3 = 𝟐𝟕. 𝟓 𝒑𝒑𝒎 𝒉𝒂𝒓𝒅𝒏𝒆𝒔𝒔 24.3 𝑔 𝑀𝑔2 + 1 𝑚𝑜𝑙𝑒 Total hardness: 51.5 𝑝𝑝𝑚 ℎ𝑎𝑟𝑑𝑛𝑒𝑠𝑠 + 27.5 𝑝𝑝𝑚 ℎ𝑎𝑟𝑑𝑛𝑒𝑠𝑠 = 𝟕𝟗. 𝟎 𝒑𝒑𝒎 𝒉𝒂𝒓𝒅𝒏𝒆𝒔𝒔 Check standards in order to determine errors for AA analysis: Table 6: Check Standards for Ca2+ Ca Concentration (ppm) Absorbance Value (at 422.7 nm) Check Standard (ppm) 0 - - 1.000 0.01717 1.45 5.000 0.05459 4.88 10.00 0.10483 10.08 25.00 0.24352 24.39 50.00 0.45635 50.40 Table 7: Check Standards for Ca2+ Mg Concentration (ppm) Absorbance Value (at 202.5 nm) Check Standard (ppm) 0 - - 1.000 0.02045 0.89 5.000 0.07657 4.40 10.00 0.1404 9.41 25.00 0.32830 24.09 30.00 0.39578 29.49 Discussion: The results in this lab show that the hardest water sample was the Kutztown creek (1.4 × 10−3 𝑀), followed by the State College tap (1.4 × 10−3 𝑀), and the Pittsburgh and Jakarta tap had equal levels of hardness (8.0 × 10−4 𝑀) which ended up being the lowest. This confirms my hypothesis, as I thought that the Kutztown creek would have be the hardest due to its lack of treatment, with each tap sample following somewhere close behind in the order of State College tap, Pittsburgh tap, then Jakarta tap. These results are as expected due to the geology around each source and the amount filtration involved in each sample’s cleaning process. As previously mentioned, because the Kutztown creek received no filtration and is located outdoors exposed to the elements, it is expected to have the highest hardness value. Next, the State College tap sample had the second highest hardness due to the large deposits of limestone found near its source. Limestone, it is known, contributes greatly to hardness due to its large concentration of calcium and magnesium ions. The Pittsburgh tap and Jakarta tap samples tied for softest water because their main sources were most polluted. Due to this extra pollution, the boroughs responsible for the cleaning of each source end up taking extra steps for filtration, resulting in the filtering out of 2+ ions (such as Ca2+ and Mg2+) that are responsible for hardness. The primary hardness levels obtained in this lab came from the EDTA titration. This is because EDTA titration is known to be more accurate than AA analysis. EDTA titration measures all 2+ ions found in the sample, while AA analysis only measures the concentrations of Ca2+ and Mg2+. When determine hardness, the EDTA titration should end up being above the AA analysis because more 2+ ions contribute to hardness rather than just calcium and magnesium. However, we can still consider AA analysis to be relatively accurate because calcium and magnesium make up the majority of hardness levels, with the other 2+ ions only contributing slightly. The results obtained for my sample of water confirms this idea, as the hardness using EDTA titration was found to be 80.0 ppm, while the AA analysis hardness was only 79.0 ppm. These hardness levels are extremely close, so we are able to confirm that both methods are relatively accurate when determining hardness levels in water samples. The softening techniques worked excellently in this lab. The baking soda softened through a method of chelation. In chelation, metal ions are combined with chemical compounds to form a ring (19). This ring then softens the water because the Ca2+ and Mg2+ ions are no longer present in ion form (19). However, this method of softening is not the best method of softening. While it did soften the samples, there is another process that softened the samples much more effectively. The more effective method of softening resulted when a resin was added to the samples. This resin worked through cation exchange (5). Cation exchanges is carried out by percolating water through a column that contains the resin (5). This resin then exchanges the calcium and magnesium ions for sodium ions (5). Sodium ions do not contribute to the water hardness at all (5). This method works extremely well, and softens each sample exponentially. As seen in the table above, every sample was softened to a point of2.0 × 10−4 𝑀, regardless of the original hardness. This method shows its effectiveness because every sample was able to be softened to the same amount just by adding a small sample of resin to each water sample. The errors that may have occurred in this lab center on the methods used. EDTA titration allows for easy, accurate titration results. However, errors still can occur. If the solution contains no Mg2+, then the color of the solution will never change and there will be no endpoint to determine the hardness of the sample. All of our samples had magnesium ions present, though, so this error did not affect us. If it did, it could simply be corrected by adding a small volume of a solution that contains the MgEDTA chelate, which reacts in water to create a useable amount of Mg2+. The AA analysis has check standards that allows an assessment of accuracy and precision as a percent error calculation. Because the hardness values obtained by AA analysis and EDTA titration are extremely close, we do not need to calculate this value for this lab because we know that both methods are very accurate. If a discrepancy had occurred between the two values, we would be able to use the check standards given in order to determine our errors in precision and accuracy. Conclusion: In conclusion, the hardest water sample was found to the Kutztown creek sample (2.8 × 10−3 𝑀), followed by the State College tap sample (1.4 × 10−3 𝑀), and the Jakarta and Pittsburgh tap samples both tied and were the least hard (each at8.0 × 10−4 𝑀). This confirmed my hypothesis that the Kutztown creek would be the hardest sample due to its location and the fact that it undergoes no treatment, and that each tap samples would vary in hardness yet be softer than the creek sample because of the simple fact that each tap sample undergoes treatment in order to lower the hardness. The softening techniques each worked as they were supposed to, and the lab generally went off without a hitch. References: 1. Good Reads. http://www.goodreads.com/ (accessed November 5, 2013). 2. The Water in You. U.S. Geological Survey. http://ga.water.usgs.gov/edu/propertyyou.html (accessed November 5, 2013). 3. Drinking Water: Hard Water. Cambridge, Ma. http://www.cambridgema.gov/CityOfCambridge_Content/documents/Drinking%20Water My%20edition.pdf (accessed November 6, 2013). 4. Hardness in Drinking Water. Water Systems Council. http://www.watersystemscouncil.org/VAiWebDocs/WSCDocs/1683274HARDNESS.PD F (accessed November 6, 2013). 5. Thompson, Stephen. PSU Chemtrek: Small-Scale Experiments for General Chemistry, 2013-2014; Hayden-McNeil Publsihing: Plymouth, MI, 2013; p 10-6. 6. Water Hardness Amounts. Office of Space Science Education. http://osse.ssec.wisc.edu/curriculum/earth/Minifact13_Water_Hardness.pdf (accessed November 6, 2013). 7. Lash, Gary George. Kutztown Quadrangle. Geologic Quadrangle Map, GQ-1577; Geological Survey (U.S.): Reston, VA, 1985. 8. Wurts, William A. Understanding Water Hardness. Universisty of Kentucky: College of Agriculture, Food and Environment. http://www2.ca.uky.edu/wkrec/Hardness.htm (accessed November 6, 2013). 9. Typical Water Well Construction and Terms. Groundwater Information Center. http://mbmggwic.mtech.edu/sqlserver/v11/help/welldesign.asp (accessed November 7, 2013). 10. State College Borough Water Authority. http://www.scbwa.org/ (accessed November 7, 2013). 11. What is my Water Hardness? Quality Water Treatment. http://www.qualitywatertreatment.com/city_water_guide.htm (accessed November 7, 2013). 12. The Pittsburgh Water & Sewer Authority 2012 Annual Drinking Water Quality Report. The Pittsburgh Water & Sewer Authority. http://apps.pittsburghpa.gov/pwsa/2012_Water_Quality_Report.pdf (accessed November 7, 2013). 13. Delinom, Robert M. Groundwater Management Issues in the Greater Jakarta Area, Indonesia. Terrestrial Environment Research Center. http://www.suiri.tsukuba.ac.jp/pdf_papers/tercbull08s2/t8supple2_46.pdf (accessed November 7, 2013). 14. Household Water Supply and Treatment Systems. Living in Indonesia. http://www.expat.or.id/info/watertreatment.html (November 7, 2013). 15. Colligan, Cory, Chem 111 Laboratory Notebook pg. 34-39. 16. Conti, Alexandria, Chem 111 Laboratory Notebook pg. 42-47. 17. Coleman, Meaghan. Chem 111 Laboratory Notebook pg. 35-39. 18. Collier, Chris, Chem 111 Laboratory Notebook pg. 40-44. 19. Wight, Chuck. How do Water Softeners Work? Scientific American. http://www.scientificamerican.com/article.cfm?id=how-do-water-softeners-wo (accessed November 7, 2013).