chem - Eradicating Zebra Mussels from Freshwater Bodies
advertisement

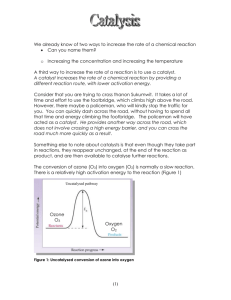
Ozone has been seen as an environmentally friendly and more efficient replacement for chlorine, however high cost and volatility of the O3 molecule have made this treatment unpopular. Ozone produces OH- and HO2 as free radicals during decay; these radicals undergo an oxidation reaction with the tissue of target organisms destroying them.1,2 In laboratory testing ozone was found to be as effective at killing mussels as chlorine and worked much quicker 5 hours compared to 18 for chlorine.3 A second experiment found that this speed advantage decreased at higher temperatures (above 30°C) as chlorine and ozone became similar in time to 95% mortality.4 O3 created by exposing air to electricity; the electric charge provides energy which causes the O2 molecules in the air to reform as O3.5 Ozone has a fast rate of decay and must be made on site for the chemical to be used.6 The fact that ozone decays rapidly means that it is safe to use in freshwater as it will not have long term toxic effects on the environment.7 Ozone is currently very expensive compared to other treatment possibilities as it can cost ten times more than chlorine treatments.8 While ozone could be an effective alternative to chlorine treatments high start up costs and the necessity for onsite production make ozone an undesirable alternative at this time. Bromine has been considered as a possible substitution for chlorine as it is also a halogen. Bromine is a liquid in its natural state making it easy to transport.9 It is produced by treating seawater with chlorine gas and air.10 Bromine can remain present much longer in an aquatic system than ozone as it degrades much more slowly.11 It reacts much the same way as chlorine destroying organic matter through an oxidation reaction. Chlorine was found to be more effective at killing larval mussels than bromine in an intermittent exposure test with 85% mortality compared to 67%.12 Bromine is potentially more effective than chlorine in high ph conditions (greater than 8.0).13 Bromine has difficulty killing adult mussels as they will close when they sense the oxidant; this makes bromine more suitable as a preventative measure than as a solution.14 Long term toxic effects for bromine are also a concern; bromine is slower to decay than chlorine and as such can remain in the water system and threaten no target species.15 Bromine does not work as well as chlorine to kill mussels and as such has not become widely used.16 Potassium permanganate is another oxidizing agent that we considered as a possible chlorine replacement. Potassium permanganate is a natural solid and is easy to move to different sites for 1 2 3 4 http://www.ozomax.com/pdf/capabilities.pdf http://www.seagrant.noaa.gov/newsevents/stories/Ballast_water_battles.html http://www.sciencedirect.com.ezproxy.lib.ucalgary.ca/science/article/pii/004313549390166F# http://www.sciencedirect.com.ezproxy.lib.ucalgary.ca/science/article/pii/S0043135496003211 5 http://www.ozomax.com/pdf/capabilities.pdf http://www.ozomax.com/pdf/capabilities.pdf 7 http://www.seagrant.noaa.gov/newsevents/stories/Ballast_water_battles.html 8 http://www.nytimes.com/1992/12/06/nyregion/in-the-war-against-zebra-mussels-the-front-is-gettingcloser.html?pagewanted=all&src=pm 9 http://www.webelements.com/bromine/ 10 http://termine.com/archives/542 11 http://www.seagrant.noaa.gov/newsevents/stories/Ballast_water_battles.html 12 http://www.springerlink.com.ezproxy.lib.ucalgary.ca/content/lu55365409722723/fulltext.pdf 13 http://el.erdc.usace.army.mil/elpubs/pdf/98e176.pdf 14 http://search.proquest.com.ezproxy.lib.ucalgary.ca/docview/221072344 15 http://el.erdc.usace.army.mil/elpubs/pdf/98e176.pdf 6 16 http://www.springerlink.com.ezproxy.lib.ucalgary.ca/content/lu55365409722723/fulltext.pdf possible deployment.17 Field preparation creates no secondary waste products and has a relatively small environmental impact compared to other treatments.18 It is worth noting that permanganate is also much more expensive to purchase than bulk chlorine 3500$/ton versus 233.75$/ton in 1997.19,20 The major drawback of permanganate is that it only kills muscles with high concentrations over a long period of time.21 Permanganate also works through an oxidation reaction. 22 Potassium permanganate was found to be generally ineffective to kill mussels compared to chlorine being both slower and requiring higher chemical concentration.23 While potassium permanganate is more environmentally friendly low toxicity against zebra mussels and high cost mean it has not been widely used as a treatment. 17 http://www.epa.gov/ogwdw/mdbp/pdf/alter/chapt_5.pdf http://www.epa.gov/ogwdw/mdbp/pdf/alter/chapt_5.pdf 19 http://www.epa.gov/ttnecas1/regdata/EIAs/Chlorine%20EIA.pdf 20 http://www.epa.gov/ogwdw/mdbp/pdf/alter/chapt_5.pdf 18 21 http://www.jstor.org.ezproxy.lib.ucalgary.ca/stable/41293583?&Search=yes&searchText=mussel&searc hText=chemical&searchText=prevention&searchText=zebra&list=hide&searchUri=%2Faction%2FdoBasic Search%3FQuery%3Dzebra%2Bmussel%2Bchemical%2Bprevention%26gw%3Djtx%26acc%3Don%26prq %3Dzebra%2Bmussel%2Bchemical%2Bsolution%26Search%3DSearch%26hp%3D25%26wc%3Don&prevS earch=&item=7&ttl=111&returnArticleService=showFullText 22 23 http://www.epa.gov/ogwdw/mdbp/pdf/alter/chapt_5.pdf http://el.erdc.usace.army.mil/elpubs/pdf/98e176.pdf
![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)