Acid-Base Equilibria
advertisement

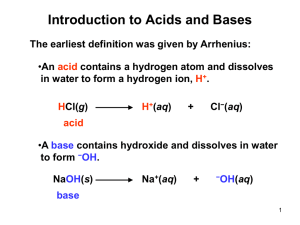
4.7: Acid-Base Equilibria show that you understand the ideas about acidity developed in the nineteenth and twentieth centuries from generalisation about substances with a sour taste to the theory that acids produce an excess of hydrogen ions in solution (Arrhenius) and then to the Brønsted–Lowry theory show that you understand that according to the Brønsted–Lowry theory: i) an acid is a proton donor ii) a base is a proton acceptor iii) acid–base equilibria involve the transfer of protons show that you understand the Brønsted–Lowry theory of acid–base behaviour, and are able to identify conjugate acid–base pairs Historical Jabir ibn-Hayyan(c722-c815) The mineral acids that we know today were discovered by the Arab alchemist. His pioneering experimental techniques and inventions of equipment are still commonly used in laboratories. He used crystallization and distillation to discover sulphuric, hydrochloric and nitric acid. He also studied ethanoic acid in vinegar and tartaric acid in wine. Antoine Lavoisier(1743-1794) and Humphry Davy Lavoisier, a French nobleman, laid the foundations of modern chemical theory. His experiments led to the oxygen theory of burning. The name oxygen means ‘acid former’ he gave the gas this name because he thought that all acids contained oxygen. However this was disproved by Davy(1809-1810) when he heated hydrogen chloride to high temperatures with a range of metals and non metals. He could find no trace of oxygen in the compound. After further work(1816) he proposed that all acids have hydrogen in common! Svante Arrhenius In 1884 the young Swede in his 20’s wrote a doctoral thesis proposing that some compounds are ionized in solution all the time. He was bitterly disappointed when he was awarded the bottom grade for his paper! In 1903 he was vindicated and awarded the Nobel prize for chemistry. He used his ionic theory to explain why all acids have similar properties when dissolved in water. His theory could account for what happens when an acid is neutralized by an alkali and could explain the difference between a strong and weak acid. In 1887 he defined an acid as a compound that could produce hydrogen ions when dissolved in water and an alkali as a compound that could produce hydroxide ions in water. HA(aq) ↔ H+(aq) +A-(aq) and an alkali as a compound that could produce hydroxide ions in water. B(aq) +H2O(aq) ↔ BH+(aq) + OH-(aq) According to his theory: Hydrochloric acid is a strong acid which is fully ionized when dissolved in water. HCl(aq) H+(aq) + Cl-(aq) Ethanoic acid is a weak acid which is only slightly ionized CH3COOH(aq) ↔ CH3CO2-(aq) + H+(aq) His theory could account for the typical reactions of dilute acids in water eg Acids + metals salt + hydrogen Acids + carbonates Acids + bases salt + carbon dioxide + water salt + water When exactly equal volumes of hydrochloric acid and sodium hydroxide of the same molar concentrations are mixed together they form a solution that has no effect on litmus paper. We say that the acid has neutralized the base or vice versa. According to Arrhenius’ theory neutralization occurs because the number of hydrogen ions (H+) is exactly equal to the number of hydroxide ions(OH-) and the two react with each other to form water(H2O) H+ (aq) + OH-(aq) H2O(aq) His theory is still useful but limited to aqueous solutions. The oxonium ion (H3O+) Once we had a greater understanding of the structure of the atom it was inconceivable that the hydrogen ion could exist independently. The H+ ion is really a proton with a diameter 70 000 times smaller than a Li+ ion! It was suggested that a H+ ion exists in association with a water molecule as the H3O+ ion, the oxonium ion. When talking about acids and bases we sometimes refer to hydrogen ions as protons we should refer to them as oxonium ions and write H3O+ instead of H+. The importance of water in the behavior of acids was recognized from observations of hydrogen chloride(HCl) and ethanoic acid(CH3COOH) dissolved in organic solvents….they do not conduct electricity and do not effect dry litmus paper….since the solution does not contain H+ ions. The Bronsted-Lowry Definition of acids and bases The preferred theory for discussing acid base equilibria was put forward by the Danish chemist Johannes Bronsted and the English chemist Thomas Lowry (1923). They described acids as proton donors and bases as proton acceptors. In aqueous solution ammonia (a base) and hydrogen chloride (an acid) react to form a solution of ammonium chloride (a salt). However the reaction between ammonia and hydrogen chloride does not need water or any other solvent to happen. The white fumes are tiny crystals of ammonium chloride, formed as ammonia gas and hydrogen chloride react together. NH3(g) + HCl(g) NH4Cl(s) This is clearly the same reaction as the one that occurs in water and ought to be an acid base reaction. However the Arrhenius definition does not allow us to do this as there is no reaction between H3O+ and OH-. From the Bronsted- Lowry definition the HCl can be seen as a proton donor and the NH3 molecule as a proton acceptor. NH3(g) + HCl(g) NH4+Cl-(s) Acid or Base Classically described by their taste as sour…..acid and soapy feel…..bases. The taste and feel test are not recommended as strong acids and bases can cause considerable harm! Some substances can act as acids and bases eg: water It acts as an acid when it reacts with substances like ammonia donating a proton during the reaction: H2O(l) + NH3(aq) OH-(aq) + NH4+(aq) It behaves as base when it reacts with hydrogen chloride accepting a proton in the reaction H2O(l) + HCl(aq) H3O+(aq) + Cl-(aq) Acids as proton donors According to Bronsted-Lowry, hydrogen chloride molecules give hydrogen ions (protons) to water molecules when they dissolve in water producing hydrated hydrogen ions called oxonium ions. The water acts as a base. Hydrogen chloride is a strong acid…..it readily gives up its protons to water molecules and its equilibrium lies well to the right effectively making it completely ionized in solution. Other strong acids are sulphuric and nitric acid. Ethanoic acid is a weak acid it is not completely ionized in water an equilibrium is established CH3COOH(aq) + H2O(aq) ↔ CH3COO-(aq) + H3O+(aq) Bases as proton acceptors According to Bronsted-Lowry, a base is a molecule or ion that can accept a hydrogen ion from an acid. A base has a lone pair of electrons which can form a dative covalent bond with a proton. An ionic oxide such as calcium oxide reacts completely with water to from calcium hydroxide . The calcium ions do not change; but the oxide ions, which are powerful proton acceptors, all take the protons from the water molecules. An oxide ion is a strong base as well as hydroxide ions, ammonia, amines, carbonates and hydrogen carbonate ions. Conjugate acid-base pairs From the Bronsted Lowry definition when an acid dissociates we can consider it as a acid-base reaction which is in equilibrium HA(aq) + H2O(aq) ↔ H3O+(aq) +A-(aq) In the forward reaction HA acts as an acid, donating a proton to a water molecule. Water accepts a proton and acts as a base. In the reverse reaction, the H3O+ acts as an acid, donating a proton to Awhich in turn is acting as a base. In acid-base reactions it is always possible to find 2 acids: HA and H3O+ and 2 bases: H2O and AIn each case, the acid on one side of the equation is formed from the base on the other side of the equation. They are called acid-base conjugate pairs. HA is the conjugate acid of A-, which means that A- is the conjugate base of HA etc. NH4+(aq) + H2O(aq) ↔ A1 B2 NH3(aq) + H30+(aq) B1 A2 The equilibrium involves 2 conjugate acid-base pairs. The p H scale