hematological disorders
advertisement

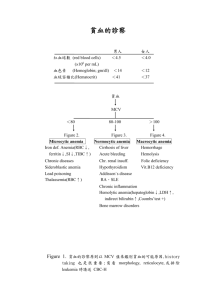
HEMATOLOGICAL DISORDERS Hematopoiesis It is the process, which maintains lifelong production of hematopoietic blood cells. The main site of hematopoiesis: o In fetal life: the liver o Throughout postnatal life: the bone marrow All hematopoietic cells are derived from pluripotent hematopoietic stem cells Erythropoiesis Erythropoiesis begins with stem cell called hemocytoblast, which is then converted to proerythroblast. Development and differentiation to stage of reticulocyte takes 4-5 days. Then erythrocyte achieves its mature form in 45-70 hours in blood Characteristics of erythrocytes • Diameter: 7,5 micrometer • No nucleus • Biconcave disk • 95% of its mass is hemoglobin • Physical properties: soft, flexible, elastic Fetal Hemoglobin Fetal hemoglobin has a higher affinity for oxygen – necessary for relatively hypoxic environment of the fetus. Hematological values at birth and the first few weeks of life • At birth, the Hb in term infants is high, 14-21.5g/dl, to compensate for the low oxygen concentration in the fetus. • The Hb falls over the first few weeks, due to reduced erythropoiesis, reaching a nadir of 10 g/dl at 2 months of age • Preterm babies have a steeper fall in Hb to 6.5-9 g/dl at 4-8 weeks of age. • Normal blood volume at birth: 80 ml/kg; in preterm infants 100 ml/kg. • White blood cell counts in neonates are higher than in older children (10-25 × 109/L). • Platelet counts at birth – as in adults (150-400 × 109/L). Anemia • Anemia is defined as a decrease of hemoglobin (Hb) level below the normal range for a child of that age and gender. • The normal range varies with age, so that normal values in pediatrics must be correlated with the patient’s age. Anemia can be defined: • Neonate: Hb <14 g/dl • 1-12 months: Hb <10 g/dl • 1-12 years: Hb <11 g/dl. Mechanism The type of anemia defines its pathophysiologic mechanism and its essential nature, allowing for appropriate therapy. • Impaired/reduced red cell production - either due to ineffective erythropoiesis (e.g. iron deficiency) or due to red cell aplasia • Increased red cell destruction (excessive hemolysis) • Blood loss - relatively uncommon cause in children unless traumatic • Blood loss should be the first consideration. Once it is ruled out, only the other two mechanisms need to be considered. • RBC survival is 120 days - maintenance of RBC population requires daily renewal of 1/120 of the cells. • Complete cessation of erythropoiesis results in a decline of about 10%/wk (1%/day) of RBCs. When RBC values fall >10%/wk without blood loss, hemolysis is a causative factor. Classification according to the Blood Film • Microcytic: Red blood cells have a low mean cell volume (MCV), appear small and pale • Macrocytic: A large MCV, appear large and oval shaped • Normocytic: Red blood cells may be normal in size and shape but may be reduced in number Microcytosis Anything that impairs hemoglobin production (Deficient or defective heme or globin synthesis) Clinical manifestation: - The clinical manifestations vary with the age, degree and rapidity of onset, presence of subjacent illness and other factors. - Mild anemia is often asymptomatic (until Hb below 6-7 g/dL) - The main consequence of anemia is tissue hypoxia. If anemia has developed rapidly, there may not be adequate time for compensatory adjustments to take place, so there is a sudden marked contraction of intravascular volume, resulting in postural hypotension, fall in cardiac output, shunting of blood from skin to central organs, sweating, restlessness, thirst and air hunger. - If anemia has slowly installation, many adaptations for the oxygen maintenance occur, such as an increase of plasmatic volume and right shift of the oxygen-hemoglobin dissociation curve. Symptoms: - Pallor (color of skin, palms, oral and conjunctival mucous membrane and nail beds) - Fatigue - Apathy - Dyspnea - Anorexia - Headache - Pica (consumption of non-food substances such as ice, chalk, ashes or clay, frequently found in iron deficiency anemia) - Bowel disturbance - Tachycardia - Syncope (particularly following exercise) - Wide pulse pressure, bounding pulse. - Headache - Syncope - Tinnitus or vertigo - Irritability Complication: Pediatric anemia may result in life-long deficits: o Negative effects persist despite correction of anemia Motor Effects: o Decreased gross and fine motor coordination Cognitive effects: o Lower scores on intelligence testing o Long-term functional impairment in school Behavioral effects: o Fearfulness and unhappiness o Early fatigue, less playful, clingy Physical Examination: Physical findings can include: o Jaundice o Splenic enlargement o Neurologic symptoms (loss of vibration sensibility, abnormal march) o Tongue atrophy o Lymphadenopathy o Evidence of blood in feces or vomit o Petechiae o Myriad signs of primary diseases that may cause a secondary anemia Laboratory Findings: o Complete blood count (with reticulocyte count, platelet count, mean cellular volume-VCM- and differential leukocyte count). o Peripheral blood smear can confirm size and color of RBC, particularly important in evaluating a patient with hemolysis. o Serum ferritin level, serum iron, and total iron binding capability (TIBC). o Bone marrow aspiration is useful and critical in the workup of any unexplained anemia, especially in underproduction anemia. Acute Post-hemorrhagic anemia o Because marrow reserve is limited, anemia may result from massive hemorrhage associated with spontaneous or traumatic rupture or incision of a large blood vessel, erosion of an artery by lesions (e.g., peptic ulcer, neoplasm), or failure of normal hemostasis. o Immediate effects depend on the duration and volume of hemorrhage. Sudden loss of 1/3 of blood volume may be fatal, but as much as 2/3 may be lost slowly over 24 h without such a risk. o Symptoms are caused by a sudden decrease in blood volume (hypovolemic shock) and by subsequent hemodilution with a decrease in the oxygen-carrying capacity of the blood. Symptoms and Signs o Faintness o Dizziness o Thirst o Sweating o Weak and rapid pulse o Rapid respiration (at first deep, then shallow) o Orthostatic hypotension is common. o Blood pressure may at first rise slightly, because of reflexive arteriolar constriction, and then it gradually falls. o If bleeding continues, blood pressure may fall and death may ensue Laboratory Findings During and immediately after hemorrhage, the RBC count, Hb, and Hct may be deceptively high because of vasoconstriction. Within a few hours, tissue fluid enters the circulation, resulting in hemodilution and a drop in the RBC count and Hb proportional to the severity of bleeding. The resultant anemia is normocytic. Polymorphonuclear granulocytosis and a rise in platelet count may occur within the first few hours. Several days after the bleeding event, regeneration (ie, reticulocytosis) is evident: Blood smears may disclose polychromatophilia and slight macrocytosis. If hemorrhage was massive and acute, occasional normoblasts and immature WBCs may be seen. Abnormal RBC production Microcytic anemia: o Iron deficiency o Thalassemia o Sideroblastic anemia Macrocytic anemia (megaloblastic): o Folate and vitamin B12deficiency Normocytic anemia: o Aplastic and Hypoplastic Anemia o Anemia of chronic disease Anemia – Reduced RBC production Etiology: 'Ineffective erythropoiesis'- red cell production occurs at a normal or increased rate but differentiation or survival of the red cells is defective (e.g. iron deficiency). Complete absence of red cell production (red cell aplasia). Diagnostic clues to ineffective erythropoiesis are: Normal reticulocyte count Abnormal mean cell volume (MCV) of the red cells: o Low in iron deficiency and high in folic acid deficiency or myelodysplasia. Iron Deficiency Anemia – Etiology: I. Inadequate intake - dietary (milk) II. Increased demand: Low birth weight Prematurity Low birth weight Multiple births High growth rate Adolescence Pregnancy III. Blood loss Perinatal o Placental Trans-placental bleeding into maternal circulation Intra-placental Fetal blood loss at or before birth Fetofetal o Umbilicus Ruptured umbilical cord Inadequate cord tying Post-exchange transfusion Postnatal: o Gut: o Primary anemia resulting in gut alteration with blood loss aggravates existing iron deficiency o Hypersensitivity to cow milk protein; exudative enteropathy o Anatomic gut lesions – varices, hiatus hernia, ulcer, ileitis Meckel’s diverticilum, hereditary teleangiectasia, o Gastritis (from aspirin ingestion, adenocortical steroids) o Henoch – Schonlein purpura o Nose: recurrent epistaxis o Uterus: menstrual loss o Kidney: Hematuria, Nephrotic syndrome (urinary loss of transferrin) Noctural Hemoglobinuria o Hemodialysis o Trauma IV. Impaired absorption: • Malabsorption syndrome • Celiac disease • Severe prolonged diarrhea • Inflammatory bowel diseases • Short bowel syndrome Iron Deficiency Anemia – Risk Factors Early cow’s milk intake Late solid food intake Frequent tea intake (tannin inhibits absorption) Low vitamin C intake Low meat intake Breast-feeding for more than 6 months without iron-reach foods or supplements Low socioeconomic status (frequent infections) Iron Deficiency Anemia – Blood Count Hemoglobin is below the lower limit of normal for age The reticulocyte count is usually normal Thrombocytopenia is more common in severe iron-deficiency anemia Thrombocytosis is present, when there is associated bleeding from the gut Low serum ferritin Serum iron and iron binding capacity: - Decreased serum iron - Increased iron binding capacity Iron Deficiency – Tissue Effect I. Gastrointestinal tract Anorexia common and early o Increased proportion of low-weight percentiles o Depression of growth Atrophic Glossitis Dysphagia Reduced gastric acidity Leaky gut syndrome o Guaiac-positive stools o Exudative eneropathy: gastrointestinal loss of protein, albumin immuno-globulins , copper , calcium, red cells Malabsorption syndrome o Iron only o Generalized Malabsorption: Xylose, Fat, Vitamin A, Duodeno-jejunal mucosal atrophy II. Central nervous system • Irritability • Fatigue • Conduct disorders • Lower mental and motor development • Papilledema III. Cardiovascular system Increase heart rate and cardiac output Cardiac hypertrophy Increase in plasma volume Increased minute ventilations values Immunologic system: There is conflicting information as to the effect on the immunologic system of iron-deficiency anemia Evidence of increased predisposition to infections o Reduction of acute illness in iron-deficient children by iron treatment and improved rate of recovery o Increased frequency of respiratory infection in iron deficiency o Impaired leukocyte transformation o Impaired granulocyte killing Evidence of decreased predisposition to infections o Lower frequency of bacterial infection in iron-deficient children Macrocystic (Megablastic) Anemia Megaloblastic anemia may result from deficiencies of Folate or B12vitamin. This deficiencies lead to impairment of DNA synthesis in rapidly growing tissue and in consequence to disproportionate maturation in the cytoplasm and nucleus during erythropoiesis. Vitamin B12 deficiency occur very rare in children Folate deficiency can occur as a result of inflammatory bowel disease, celiac disease, anticonvulsant therapy Bone marrow (ineffective erythropoiesis) Normocytic Anemia • Primary bone marrow disorders (aplasia and hypoplasia, myelodisplasia, myelofibrose, hematologic and solid tumors, HIV infection, granulomas) • Chronic disease anemia • Secondary anemia (hepatic, renal or endocrine disorders) Aplastic and Hypoplastic Anemia Anemia resulting from a loss of RBC precursors, either from a defect in stem cell pool or an injury to the microenvironment that supports the marrow, and often with borderline high MCV values. The term aplastic anemia (bone marrow failure) commonly implies a panhypoplasia of the marrow with associated leukopenia and thrombocytopenia. The term pure RBC aplasia, defines the selective marked reduction or absence of erythroid precursors. Although both disorders are uncommon, aplastic anemia is more common. Both, aplastic and Hypoplastic Anemia may be acquired or congenital Acquired Aplastic Anemia Etiology and pathogenesis: About 1/2 of the cases of true aplastic anemia are idiopathic. Recognized causes are: o Chemicals (e.g. benzene, inorganic arsenic), o Radiation o Drugs (e.g. antineoplastic, antibiotics, NSAIDs, anticonvulsants) Symptoms and signs: • Signs vary with the severity of the pancytopenia. • General symptoms of anemia are usually severe. • Waxy pallor of skin and mucous membranes. Chronic cases may show considerable brown skin pigmentation. • Severe thrombocytopenia, with bruising and bleeding. Hemorrhages into the ocular fundi are frequent. • Agranulocytosis with life-threatening infections. • Splenomegaly is absent, unless induced by transfusion hemosiderosis. Laboratory findings: • RBCs are normochromic-normocytic (sometimes marginally macrocytic). • A WBC count <= 1500/µL3 is common, the reduction occurring chiefly in the granulocytes. • Platelets are often markedly reduced. • Reticulocytes are decreased or absent, even with coexistent hemolysis. • The aspirated bone marrow is acellular. • Serum Fe is elevated. Congenital Aplastic Anemia Fanconi´s Aniemia A very rare congenital form of aplastic anemia (autosomal recessive) Presents at 5-6 years of age. Transforms into acute leukemia. Symptoms: o Bone abnormalities (radius, thumbs) o Short stature o Renal malformations o Microcephaly o Hypogonadism, and o Brown pigmentation of skin Schwachman-Diamond Syndrome A rare congenital condition (autosomal recessive) Mild pancytopenia or isolated neutropenia Transforms to acute leukemia. Symptoms: o Bone marrow failure o Pancreatic exocrine failure o Skeletal abnormalities Red cell aplasia (Hypoplastic Anemia) Etiology: o Congenital red cell aplasia (Diamond-Blackfan anemia) o Transient erythroblastopenia of childhood o Parvovirus B19 infection - only causes red cell aplasia in children with inherited hemolytic anemia’s and not in healthy children. Diagnostic Clues: • Low reticulocyte count despite low Hb • Normal bilirubin • Negative direct antiglobulin test (Coombs' test) • Absent red cell precursors on bone marrow examination Blackfan – diamond anemia o A rare congenital disease o 80% Sporadic mutation o Presentation at 2-3 months, 25% at birth o Short stature and abnormalities of the thumbs or digits o RBC may have fetal Hb. o Treatment with steroids (60-70% responders) or monthly RBC transfusions Transient erythroblastopenia of childhood (TEC) A brief reversible disappearance of RBC precursors in the marrow during various acute viral illnesses in young children. TEC is thought to be due to an abnormal response to infection, perhaps via an antibody that inhibits RBC progenitors. The RBC are normal in size and do not express fetal characteristics. Parvovirus B19 infection o Only in children with chronic hemolytic anemia, parvovirus B19 infection may cause aplastic crises (direct infection of the RBC progenitors) o Usually last 1-2 weeks, but prolonged aplasia has been described. Increased red cell destructions hemolytic - Anemia • The essential feature of hemolytic anemia is a reduced RBC lifespan (normal - about 120 days), due to increased red cell destructions in the circulation (intravascular hemolysis) or liver or spleen (extravascular hemolysis) • Hemolysis results in anemia when bone marrow production can no longer compensate for the shortened RBC survival (eightfold increase possible) Etiology: Immune hemolytic anemia – common in neonates, uncommon in elder children The main cause of hemolysis in children is intrinsic abnormalities of the red blood cells: o Red cell membrane disorders (e.g. hereditary spherocytosis) o Red cell enzyme disorders (e.g. glucose-6-phosphate dehydrogenase deficiency) o Hemoglobinopathies (abnormal hemoglobin, e.g. βthalassemia major, sickle cell disease). Increased red cell breakdown lead to: Anemia Reticuloendothelial hyperplasia - hepatomegaly and splenomegaly Elevated unconjugated bilirubin Excess urinary urobilinogen. Diagnosis Raised reticulocyte count (on the blood film this is called 'polychromasia' as the reticulocytes have a characteristic lilac color) Unconjugated bilirubinaemia and increased urinary urobilinogen Abnormal appearance of the red cells on a blood film (e.g. spherocytes, sickle shaped or very hypochromic) Positive direct antiglobulin test – Coombs’ test identifies antibodycoated red blood cells (positive only if an immune cause of hemolytic anemia) Increased erythropoiesis in the bone marrow. Hereditary spherocytosis • 1 in 5000 births in Caucasians • An autosomal dominant inheritance, 25% caused by new mutations • Mutations in genes for the skeletal proteins of the red cell membrane (mainly spectrin, ankyrin or band 3). • Reduction in red cells surface-to-volume ratio causes the cells to become spheroidal • RBC are less deformable – what leads to their destruction in the microvasculature of the spleen. Symptoms: Jaundice - usually develops during childhood but may be intermittent; (may cause severe hemolytic jaundice in the first few days of life) Anemia - mild anemia (hemoglobin 9-11g/dl) - The hemoglobin level may fall with an intercurrent infection - Many children have 'compensated' hemolysis with a normal hemoglobin Mild to moderate splenomegaly - depends on the rate of haemolysis; o This may be the mode of presentation in the first year of life Aplastic crisis - uncommon, associated with parvovirus B19 infection Gallstones - due to increased bilirubin excretion. Diagnosis: • The blood film usually diagnostic • Specific tests available (e.g. osmotic fragility) Differential diagnosis: Antibody-induced anemia may be associated with spherocytosis. It must be excluded with a direct antiglobulin test especially in the absence of a family history. Glucose -6 – phosphate dehydrogenase (G6PD) deficiency • The most common red cell enzymopathy • Over 100 million people are affected • High prevalence (10-20%) in central Africa, the Mediterranean, the Middle East and the Far East. • Many different mutations - different clinical features • G6PD is essential for preventing oxidative damage to red cells. Red cells lacking G6PD are susceptible to oxidant-induced haemolysis. • X-linked inheritance – males predominantly affected (10-15% normal enzyme activity) • Females - heterozygotes – 50% of the normal G6PD activity. Symptoms: o Neonatal jaundice: In the first 3 days of life - it is the most common cause of severe neonatal jaundice requiring exchange transfusion. o Acute haemolysis - precipitated by: o Infection, the most common precipitating factor o Certain drugs (antimalarials, sulphonamides, quinolones, high dose aspirin) o Fava beans (broad beans) o Naphthalene in mothballs Diagnosis: o Measuring G6PD activity in red blood cells o Misleadingly elevated enzyme level during hemolytic crisis – Young red blood cells may have normal enzyme activity whilst older cells are deficient - a need for repeated assay Hemoglobinopathies Red blood cell disorders which cause hemolytic anemia because of: Reduced or absent production of HbA (α- and β-thalassemia) or Production of an abnormal Hb (e.g. sickle cell disease). α-Thalassemia are caused by deletions in the α-globin gene. β-Thalassemia and sickle cell disease are caused by mutations of the β-globin gene. Clinical manifestations of the Hemoglobinopathies affecting the β-chain are delayed until after 6 months of age when most of the HbF present at birth has been replaced by adult HbA Sickle cell disease The most common genetic disorder in children in many European countries, (prevalence 1 in 2000 live births) - neonatal screening using the biochemical screening test (Guthrie test) Hemoglobinopathies in which HbS is inherited. HbS forms as a result of a point mutation in codon 6 of the β-globin gene Most common in patients from tropical Africa or the Caribbean, is also found in the Middle East and in low prevalence in most other parts of the world except for northern Europeans Pathogenesis: HbS molecule becomes deformed (insoluble) in the deoxygenated state. HbS polymerises within red blood cells forming rigid tubular spiral bodies, which deform the red cells into a sickle shape. Irreversibly sickled red cells have a reduced lifespan and may be trapped in the microcirculation, resulting in thrombosis and therefore ischemia in an organ or bone. This is exacerbated by low oxygen tension, dehydration, cold, excessive exercise or stress. The clinical manifestations vary widely between different individuals. Symptoms o Anemia (6-8 g/dl)with jaundice o Infection (pneumococcal or H. influenza) due to hyposplenism secondary to micro-infarction in the spleen) o Painful vaso-occlusive crisis: in any organ (hand-foot syndrome with dactylitis), cerebral and pulmonary infarction less common but serious (stroke) o Acute anemia: Hemolytic, aplastic or sequestration crises from accumulation of sickled cells in spleen o Priapism o Splenomegaly o Long term problems: cognitive problems, heart failure, cardiac enlargement, renal dysfunction, gallstones, leg ulcers, short stature and delayed puberty B-Thalassemia In people from the Indian subcontinent, Mediterranean and Middle East As there is a deficiency of β-chains, γ-chain synthesis continues beyond the neonatal period, producing an increased proportion of HbF, and δ-chain production increases the amount of HbA2. This results in precipitation of globin chains within the red cell membrane, bringing about cell death within the bone marrow (ineffective erythropoiesis) and premature removal of circulating red cells by the spleen. The disease severity of β-thalassaemia depends on the amount of HbA and HbF present: o β-Thalassemia major - HbA (α2β2) cannot be produced because of the abnormal β-globin gene. The most severe form. o β-Thalassemia intermedia - the β-globin mutations allow a small amount of HbA and/or a large amount of HbF to be produced. Milder, variable. o β-Thalassemia trait - one normal and one abnormal β-globin gene. Asymptomatic carrier Symptoms: Severe anemia and jaundice from 3-6 months of age Failure to thrive/growth failure Extra-medullary hematopoiesis, causing bone marrow expansion (classical feces with maxillary overgrowth and skull bossing) Marked hepatosplenomegaly (not seen in appropriately transfused children). Blood Transfusion RBC lifelong monthly transfusions The complications of multiple transfusions: o Chronic iron overload: causes cardiac failure, liver cirrhosis, diabetes, infertility and growth failure - iron chelation therapy with subcutaneous desferrioxamine, given overnight from 2 to 3 years of age o Allo-antibody formation – problems with compatible blood o Infections (HIV, Hepatitis A, B, C, malaria – rare) o Problems with venous access Bone marrow transplantation if possible Healthy individuals have four α-globin genes. The manifestation of α-thalassemia syndromes depends on the number of functional αglobin genes. α-thalassemia major (Hb Barts hydrops fetalis) - deletion of all four α-globin genes, so no HbA (α2β2) can be produced. It presents in mid-trimester with fetal hydrops (edema and ascites) from fetal anaemia, which is always fatal in utero or within hours of delivery. When only three of the α-globin genes are deleted (HbH disease), affected children have mild-moderate anemia Anemia in neonates Symptoms: Pallor Air hunger Hypotension or shock In extreme cases, heart failure and hydrops may develop Etiology: Prenatal factors: o Placenta praevia o Cord rupture o Feto-maternal or feto-placental bleeding o Twin-twin transfusion Postnatally: o Hemorrhage in the neonate, for example as a result of trauma or coagulopathy o Iatrogenesis - phlebotomy in sick neonates, lab test Increased breakdown may be the result of: o Hemolytic disease of the newborn Hemolytic disease of the newborn: Hemolytic disease of the newborn is diagnosed when hemolysis of a neonate's red blood cells is caused by antibody from the mother. This condition only occurs where there is incompatibility between the maternal and fetal blood. Etiology: o One of the most severe causes of this condition is rhesus (Rh) incompatibility. o Other causes include, in descending order of frequency and severity: - AB0 system incompatibility - Red blood cell metabolic disorders - G-6-PD (glucose-6-phosphate dehydrogenase) deficiency - Pyruvate kinase deficiency - RBC morphology disorders - for example, hereditary spherocytosis, o Hereditary elliptocytosis o Rhesus incompatibility tends to be more severe than AB0 incompatibility because anti-D antibodies are mainly IgG, which can easily cross the placenta, whereas anti-A and anti-B antibodies are mainly IgM, which cannot cross the placenta. Investigation If hemolytic disease of the newborn is suspected, the following investigations should be carried out: o Full blood count, with attention to hemoglobin, white cells and reticulocytes o Platelets o Infant blood group and Coombs test o Maternal blood group and hemolysis o Red cell enzyme assay may be a helpful second line investigation o Blood film and osmolar fragility may diagnose spherocytosis