HW 4-Spring 2010
advertisement

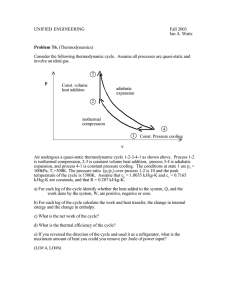
CHEN 354- HW # 4- Due Friday, February 19, 2010 Problem 1. Methane, hydrogen, and propane, all ideal gases, are mixed together in equal parts by mass to create a new fuel gas. The gas is then adiabatically compressed from 10oC to 0.1 m3 at 2 MPa, 25oC. Determine the work required for the compression process. Solution: Given: mfCH4 = mfH2 = mfC3H8 = 0.333. (Equal parts by mass.) T1 = 10oC, V2 = 0.1 m3, p2 = 2 MPa, T2 = 25oC. The process is adaiabatic (Q = 0), and we will assume that there is no change in kinetic or potential energy. Therefore, for this closed system, the first law gives W = -m (u2 - u1) = m cv (T2 - T1) The constant volume specific heat of the mixture, is the sum of the mass fractions of each components times their specific heat of each component. If we assume that the gases have constant specific heats, cv = [(mf)(cv)]CH4 + [(mf)(cv)]H2 + [(mf)(cv)]C3H8 cv = (0.333)(1.709) + (0.333)(10.19) + (0.333)(1.502) = 4.46 kJ/kg-K The molecular mass of the mixture can be found by taking the inverse of the sum of the mass fractions divided by the molecular mass of each component. This yields M = 5.13 kg/kmole. The gas specific ideal gas constant is then R = Ru/M = 1.62 kJ/kg-K. The mass of the mixture can then be found from the ideal gas law at the second state: m = pV/RT = (2000 kPa)(0.1 m3)/((1.62 kJ/kg-K)(298 K) = 0.414 kg. The work used to compress the gas is then W = - (0.414 kg)(4.46 kJ/kg-K)(25 - 10)K = -27.7 kg