Fluidized Bed Theory (RK) (AG)
advertisement

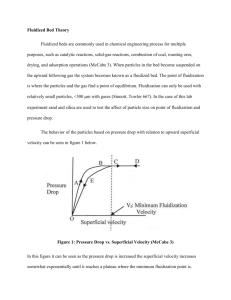
Fluidized Bed Theory Fluidized beds are commonly used in chemical engineering process for multiple purposes, such as catalytic reactions, solid-gas reactions, combustion of coal, roasting ores, drying, and adsorption operations (McCabe 3). Even though fluidized beds also contain a packing material to give good contact between the phases, they have several major differences from packed beds. First, the packing is supported by the upflowing phases and behaves much like a liquid (hence the name). The packing phase is in constant motion within the fluidized bed. Such a unit is more complex than a simple fixed bed and has several advantages which can be important in industrial applications: (1) the rapid mixing motion in the bed gives a high heat transfer rate between the bed and the shell of the unit; thus, heat can be easily transferred to or away from the bed, (2) the bed unit tends to be quite uniform in concentration when compared to the non-mixed packed bed; this can be an advantage or a disadvantage for a given separation or chemical reaction, and (3) the packing can flow out of the unit for separate treatment and back into the unit. (McCabe 145) At low gas velocities, the pressure drop through the bed can be described by the Ergun equation. However, as the flowrate increases a point is reached where the pressure drop becomes constant and does not change with gas flowrate. This is defined as the point of fluidization. The point of fluidization is where the particles and the gas find a point of equilibrium. When particles in the bed become suspended on the upward following gas the system becomes known as a fluidized bed. Fluidization can only be used with relatively small particles, <300 μm with gases (Sinnott, Towler 667). In the case of this lab experiment sand and silica are used to test the affect of particle size on point of fluidization and pressure drop. Visually, the bed does not appear like a fixed bed but there is random motion of the particles within the bed; if a valve is opened on the side of the unit the packing will flow out much like a liquid. Further increases in gas flowrate result in violent mixing within the bed and the formation of large gas bubbles passing through the bed. Upon decreasing the gas flowrate, the pressure drop versus gas flowrate does not exactly follow the previous curve as seen in figure 1 below. There is a significant hysterisis effect due to the frictional forces in the initial packed bed. The reason for this path dependence is due to a change in the orientation of the packing particles as they become looser than before due to the air flowing through the bed; this results in a lower friction factor and lower pressure drop. The behavior of the particles based on pressure drop with relation to upward superficial velocity can be seen in figure 1 below. Figure 1: Pressure Drop vs. Superficial Velocity (McCabe 3) In this figure it can be seen as the pressure drop is increased the superficial velocity increases somewhat exponentially until it reaches a plateau where the minimum fluidization point is. Along the first section of the graph (area A) the pressure drop can be related to the velocity using the Ergun Equation (equation 1 below). This equation can only be used in this section because of the small pressure drop and velocity where it is considered a packed bed (Bird, Stewart, Lightfoot 191). (𝑃0 −𝑃𝐿 )∗𝜌 ( 𝐺02 𝐷𝑝 𝜀3 𝐿 1−𝜀 )∗( )∗( 1−𝜀 ) = 150 ∗ (𝐷𝑝 𝐺0 ) + ⁄𝜇 7 4 (1) Where: P0 = initial pressure [Pa] PL = pressure at the end of the column [Pa] G0 = mass flux [kg/m2*s] Dp = particle diameter [m] L = height of the bed [m] ε = void fraction [dimensionless] ρ = density of the particle [kg/m3] μ = viscosity [kg/s*m] However as the velocity and pressure drop increase the Ergun Equation (1) cannot be used in the determination of fluidized bed values. Figure 2 below also shows this relation but with the overall bed height verses the superficial velocity in the column. Figure 2: Bed Height vs. Superficial Velocity (McCabe 3) Point C on both figures is the point of minimum fluidization velocity, Vf, and can be caluculated by the following equations. (Note: The small frictional force exerted on the wall was ignored). First the determination of the upward force by the gas on the bed can be calculated using equation 2 below. 𝑈𝑝𝑤𝑎𝑟𝑑 𝑓𝑜𝑟𝑐𝑒 𝑜𝑛 𝑡ℎ𝑒 𝑏𝑒𝑑 = ∆𝑃 ∗ 𝐴 (2) Where: ΔP = pressure drop across the bed [Pa] A = cross-sectional area of the bed [m2] Next the volume of the particles can be solved for using equation 3 below. 𝑉𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑝𝑎𝑟𝑡𝑖𝑐𝑙𝑒𝑠 = (1 − 𝜀 ) ∗ 𝐴 ∗ 𝐿 Where: (3) ε = void fraction [dimensionless] A = cross-sectional area of the bed [m2] L = height of the bed [m] From here we can determine the net weight of the particles in the column by using equation 4 below. 𝑁𝑒𝑡 𝑤𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝑡ℎ𝑒 𝑝𝑎𝑟𝑡𝑖𝑐𝑙𝑒𝑠 = (1 − 𝜀) ∗ (𝜌𝑝 − 𝜌𝑓 ) ∗ 𝐴 ∗ 𝐿 ∗ 𝑔 (4) Where: A = cross-sectional area of the bed [m2] L = height of the bed [m] ε = void fraction [dimensionless] 𝜌𝑝 = density of the particle [kg/m3] 𝜌𝑓 = density of the fluid (or gas) [kg/m3] g = acceleration due to gravity [m/s2] The theoretical pressure drop can be determined by using equation 5 below. ∆𝑃 = (1 − 𝜀 ) ∗ (𝜌𝑝 − 𝜌𝑓 ) ∗ 𝐿 ∗ 𝑔 Where: L = height of the bed [m] ε = void fraction [dimensionless] (5) 𝜌𝑝 = density of the particle [kg/m3] Typically for a bed with small particles (Dp < 0.1 mm), the flow conditions are such that the Reynolds number (Re) is relatively small (Re < 10) that the Kozeny-Carmen Equation can be used to find the velocity of fluidization (Vf) (McCabe 5). This Kozeny-Carmen Equation can be seen as equation 6 below. 𝑉𝑓 = (𝜌𝑝 − 𝜌𝑓 )∗𝑔∗𝐷𝑝2 150∗𝜇 ∗ 𝜀3 1−𝜀 (6) Where: Vf = fluid velocity [m/s] ε = void fraction [dimensionless] 𝜌𝑝 = density of the particle [kg/m3] 𝜌𝑓 = density of the fluid (or gas) [kg/m3] g = acceleration due to gravity [m/s2] Dp = particle diameter [m] μ = viscosity [kg/s*m] When the superficial velocity (Vs) is equal to the fluidized velocity (Vf) this state is known as incipient fluidization (McCabe 5). Next the settling velocity can be determined by restricting the size of the particle to be small like before so that Stokes Law can be used to calculate this velocity. This can be seen in equation 7 below. 𝑉𝑆𝑒𝑡𝑡𝑙𝑖𝑛𝑔 = (𝜌𝑝 − 𝜌𝑓 )∗𝑔∗𝐷𝑝2 18∗𝜇 (7) Where: Vsettling = settling velocity [m/s] 𝜌𝑝 = density of the particle [kg/m3] 𝜌𝑓 = density of the fluid (or gas) [kg/m3] g = acceleration due to gravity [m/s2] Dp = particle diameter [m] μ = viscosity [kg/s*m] Once the velocity of settling and the fluidization velocity are determined a ratio of the two can be formed relating the void fraction back to both the velocities, which can be seen in equation 8 below. 𝑉𝑠𝑒𝑡𝑡𝑙𝑖𝑛𝑔 𝑉𝑓 = 25 3 ∗ 1− 𝜀 𝜀3 (8) Where: Vf = superficial velocity [m/s] Vsettling = settling velocity [m/s] ε = void fraction [dimensionless] In cases where the particles a very small it is likely that they may be carried out of the bed system, therefore filters or cyclones must be emplaced to recover these particles at high superficial velocities. Bubbling fluidization will occur in this experiment because this is strictly a gas-fluidized bed which will be seen as large pockets of gas moving through the free particles. References: Bird, R. Byron, Warren E. Stewart, and Edwin N. Lightfoot. Transport Phenomena. New York: J. Wiley, 2007. Print. Sinnott, Ray, and Gavin Towler. Chemical Engineering Design. Amsterdam: Elsevier, 2009. Print. W.E. McCabe, J.C. Smith, and P. Harriott 2001. Unit Operations of Chemical Engineering, McGraw Hill, New York.