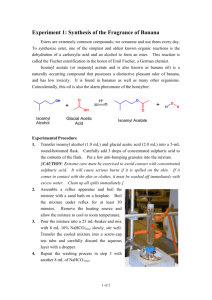
Esterification and Odors of Esters
advertisement

NCSU – Dept. of Chemistry – Lecture Demonstrations Organic Chemistry Esterification and Odors of Esters Description: An alcohol and acid are mixed together to generate an ester with a pleasant odor. Materials: Conc. H2SO4 Glacial acetic acid Salicylic acid Isoamyl alcohol Methanol Ethanol Plastic pipets Thin-stemmed pipet Small cork stoppers Beaker (for water bath) Hot plate Procedure: Cut the tip off of the plastic pipet and use this as the reaction vessel. Prepare the following three esters by combining the proper number of drops (from thin-stemmed pipet) of corresponding alcohol and acid, sealing the vessel with a cork stopper, and heating at 70-80 oC in a hot water bath for 10 minutes. After heating, carefully remove the cork stopper and waft vapors of ester to observe the smell. Ester Isoamyl acetate Ethyl acetate Methyl salicylate ROH (# of drops) Isoamyl (10) Ethanol (10) Methanol (20) Acid (# of drops) Acetic (10) Acetic (10) 0.25 g salicylic H2SO4 (# of drops) 1 1 4 The esters synthesized should have the following scents: isoamyl acetate (banana), ethyl acetate (fruity), methyl salicylate (wintergreen). A collection of other “scented” chemicals can be found with the ester synthesis kit in Dabney 114. NCSU – Dept. of Chemistry – Lecture Demonstrations Organic Chemistry Discussion: These reactions demonstrate a Fischer esterification in which an alcohol reacts with a carboxylic acid to generate an ester and water as the two products. The structures of the esters generated in this experiment are shown below. HO O O O O O isoamyl acetate O ethyl acetate methyl salicylate Safety: Wear proper protective equipment including gloves and safety glasses when preparing and performing this demonstration. Concentrated acids will cause burns so extreme care must be taken when handling these acids. Disposal: Dispose of contents in an appropriate waste container. References: Newton, T.; Rothenberger, O.; Bunting, R. J. Chem. Educ. 1991, 68, 502.