Timetable of REACH Implementation
advertisement
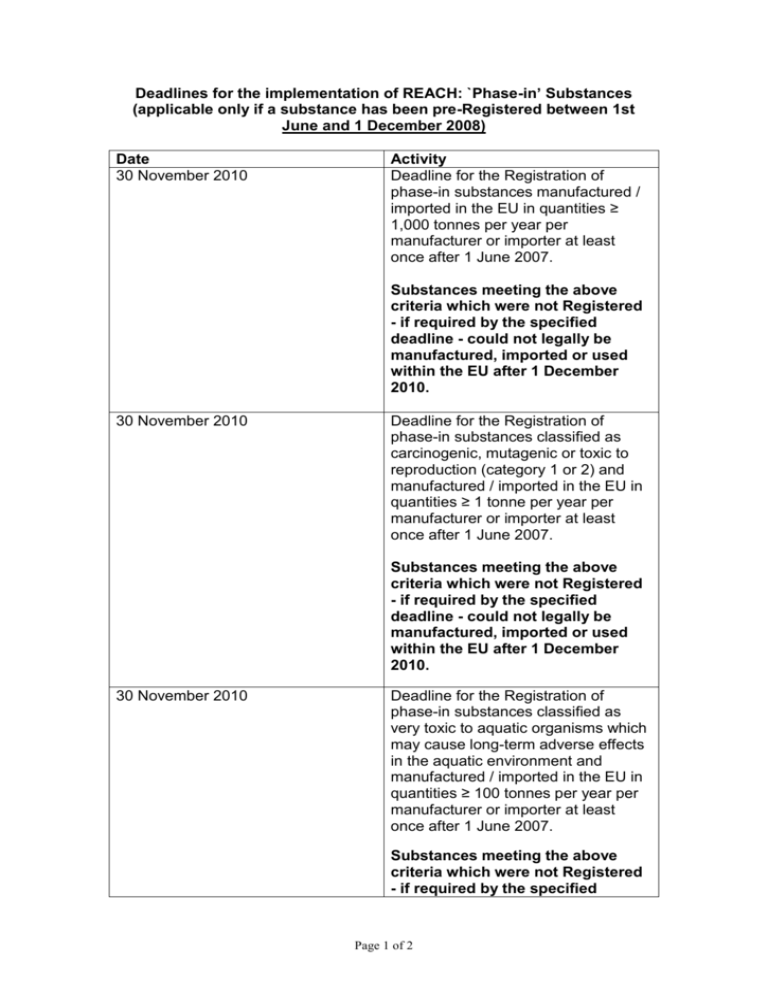
Deadlines for the implementation of REACH: `Phase-in’ Substances (applicable only if a substance has been pre-Registered between 1st June and 1 December 2008) Date 30 November 2010 Activity Deadline for the Registration of phase-in substances manufactured / imported in the EU in quantities ≥ 1,000 tonnes per year per manufacturer or importer at least once after 1 June 2007. Substances meeting the above criteria which were not Registered - if required by the specified deadline - could not legally be manufactured, imported or used within the EU after 1 December 2010. 30 November 2010 Deadline for the Registration of phase-in substances classified as carcinogenic, mutagenic or toxic to reproduction (category 1 or 2) and manufactured / imported in the EU in quantities ≥ 1 tonne per year per manufacturer or importer at least once after 1 June 2007. Substances meeting the above criteria which were not Registered - if required by the specified deadline - could not legally be manufactured, imported or used within the EU after 1 December 2010. 30 November 2010 Deadline for the Registration of phase-in substances classified as very toxic to aquatic organisms which may cause long-term adverse effects in the aquatic environment and manufactured / imported in the EU in quantities ≥ 100 tonnes per year per manufacturer or importer at least once after 1 June 2007. Substances meeting the above criteria which were not Registered - if required by the specified Page 1 of 2 deadline - could not legally be manufactured, imported or used within the EU after 1 December 2010. 31 May 2013 Deadline for the Registration of phase-in substances manufactured / imported in the EU in quantities ≥ 100 tonnes per year per manufacturer or importer at least once after 1 June 2007. 31 May 2018 Deadline for the Registration of phase-in substances manufactured / imported in the EU in quantities ≥ 1 tonne per year per manufacturer or importer at least once after 1 June 2007. Note: Non phase-in substances do not benefit from the transitional regime provided for phase-in substances and need to be Registered before they can be manufactured, imported or placed on the market in the EU, unless they have already been notified under Directive 67/548/EEC. Page 2 of 2






