Chp 17 Outdoor & Indoor Air Pollution Notes
advertisement

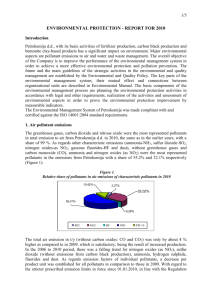
Chp 20 Outdoor & Indoor Air Pollution Notes The Earth’s Atmosphere is made up of the Troposphere (0-11 miles), the Stratosphere (11-30 miles), the Mesosphere (30-50 miles) and the Thermosphere (50-75 miles) The temperature in the troposphere decreases with increasing elevation. The same can be said of the stratosphere. In between the two layers the temperature increases so that the two layers do not mix. Where the temperature rises is termed the tropopause. Outdoor and Indoor air pollution deal with the Troposphere. Global Warming will deal with both the troposphere and the stratosphere. Ozone Depletion will deal with mostly the stratosphere. Outdoor Air Pollution Air Pollution – the presence of one or more chemicals in the air in quantities that are in excess of the normal natural concentrations Naturally occurring Pollutants VOC’s – trees (terpenes & isoprenes), animal waste (aldehydes, methanol, acetone) CH4 -- metabolic activity in organisms, decaying swamp vegetation SO2 -- volcanoes, sea spray, decaying vegetation H2S – geysers and hot springs, decay in bogs Particulates – volcanoes(ash), forest fires (ash), trees (pollen), dust storms (dirt) CO -- forest fires CO2 -- respiration of organisms N2O -- soil denitrification by bacteria NOx – decaying organic matter and the ammonia in fertilizer O3 – lightning (in the troposphere) Air Pollution Sources Point Source -– emit air pollution from a controlled site (smokestacks, vents, etc) Non-Point Sources – burning leaves, road dust, dust from construction site, etc. Fugitive Sources – open areas exposed to wind processes – dirt roads, construction sites, farmland, surface mines, etc. Mobile Sources – emitters that move from place to place – autos, trucks, buses, aircraft, ships, trains Area – containing more than one point source – i.e. several factories, a city, county, etc. Primary Air Pollutants : SO2, NO, NO2, CO, CO2, PM-10, VOC’s (including hydrocarbons) Anthropogenic (man-made) sources: SO2 produced from burning fossil fuels (mostly coal) NO & NO2 (known as NOx) produced from fossil fuel burning (mostly from car exhaust) CO produced from fossil fuel burning (mostly from car exhaust) PM-10 produced from fly ash, carbon black (soot), smoke (tobacco, oil), fumes from industry, agricultural practices (insecticide & pesticide spraying, plowing) VOC’s produced from industrial processes (solvents, dyes, finishes, etc – This includes CFCs and HCs i.e. benzene) Health Effects of Primary Pollutants: SO2, NO, NO2 – lung irritant and aggravates respiratory conditions (asthma, bronchitis, emphysema) PM-10 – nose & throat irritation, bronchitis, early death (black lung disease) VOC’s (Hydrocarbons) – lung irritant, aggravates respiratory conditions, carcinogens (in greater concentrations) CO – headaches, dizziness, binds with hemoglobin more than oxygen leading to coma and death Secondary Air Pollutants – created from primary air pollutants with sunlight & temperature as catalysts O3, SO3, H2SO4, HNO3, NO3- salts, SO42- salts, PAN’s, aldehydes Equations for formation of Secondary Air Pollutants: NO2 + UV radiation NO + O (atom); O + O2 O3 Ozone formation is a 2 step process. Natural ozone formation in the troposphere is created by lightning striking O 2 molecules. SO2 + O2 SO3; SO3 + H2O H2SO4 NO2 + H2O HNO3 + NO H2SO4 formation is a several step process. HNO3 & H2SO4 are the two acids responsible for acid deposition. NO2 + O2 + hydrocarbons PAN (peroxyacetylnitride – an eye irritant) O2 + O3 + VOC’s aldehydes (like formaldehyde – a carcinogen) Health Effects of Secondary Pollutants O3 – eye irritant, aggravation of respiratory problems, reduced resistance to viral infections PAN – eye irritant, aggravation of respiratory problems Aldehydes - eye irritant, aggravation of respiratory problems H2SO4 & HNO3 – discussed below Acid Deposition – acid (HNO3 or H2SO4) or acid derived salts (NO3- or SO42-) that fall in either a wet (rain, snow, fog, etc) or dry (suspended particles) form over the land. Any area downwind of a coal burning power plant, factory or smelter is susceptible to acid deposition. Since the US has our prevailing Westerlies, acids from the west can affect the areas in the east. Also, areas that do not have limestone (CaCO 3) soils (a natural buffer) will be more affected by acid deposition. Effects of Acid Deposition: Reduces visibility Direct effect on leaves and needles of plants – causes chlorosis,(yellowing of leaves & needles) which will destroy the chlorophyll and thus inhibit growth The nutrients (Ca2+, Mg2+) the plant needs are leached from the topsoil weakening the plant, making it susceptible to disease and pests The Al3+ that was bound up in the soil is now released and binds with the roots of the plant preventing any uptake of nutrients, weakening the plant making it susceptible to disease and pests The Al3+ washes into the stream and clogs the gills of fish (by the fish producing mucus) causing fish kills Inorganic mercury undergoes a chemical change in the acidic watery environment to methyl mercury which bioaccumulates in the fish and subsequently bioaccumulates in the humans who eat them causing brain damage or neurological disorders. Acidifies lakes and streams killing aquatic life (fish, macroinvertebrates, phytoplankton) when the pH is 4.5 and less Dissolves buildings, statues, and bridges that contain limestone or marble. (Concrete contains limestone) Solublizes toxic metals in the soil that can be absorbed by plants Promotes the growth of acidic mosses that kill trees by holding so much water that the roots drown or the soil becomes anaerobic or they kill the mycorrhizal fungi that help the plants roots absorb nutrients Slows the decomposition of plant material on the forest floor because the acid destroys the microbial decomposers Ways to REDUCE acid depositon: Improve energy efficiency or reduce energy use Reduce use of coal Increase use of renewable resources Burn low-sulfur coal (black coal – anthracite or bituminous) Remove SO2 from smokestack emissions with better wet scrubbers Remove sulfur from brown coal (bituminous and lignite) Remove NO2 from car exhaust by lowering the temperature of the exhaust Tax emissions of SO2 Add lime to acidified lakes Add phosphate fertilizer to acidified lakes Requiring the same standards of ‘grandfathered’ power plants as the newer power plants Photochemical Smog – a mixture of primary and secondary air pollutants formed under the influence of sunlight. More NO2 is created from NO during the day giving photochemical smog its characteristic brown color. NO2, O3, and PAN’s are photochemical oxidants. They can react with compounds that don’t ordinarily react with oxygen which can then irritate the respiratory tract. Formation of Secondary Air Pollutants 15 The NO changes to NO2 when it combines with O2. As the sun rises the UV light and the heat of the day catalyze the NO2 to break down and then ozone is formed. 13 Concentration 11 9 NO NO2 Ozone 7 5 3 1 6:00 AM 7 8 9 10 11 12 noon 1 2 3 4 5 6:00 PM Time of Day Industrial Smog – made up of a mixture of SO2, droplets of H2SO4, and PM-10 from coal (soot). It is also called gray smog. Factors influencing the formation of Smog: climate topography population density the amount of industry the type of fuels used in industry, heating, and transportation Anthropogenic Pollutants / Sources/ Sinks Pollutant SO2 Source burning fossil fuels Petroleum refining Paper production Cement production Aluminum production SO2 Sink soil (as H2SO4 that leaches nutrients) air (making H2SO4) NO2 Automobiles Coal burning Plants NO2 air (as N2 or NO3-) stratosphere (as substrate for ozone depletion) soil (as HNO3 that leaches nutrients) soil (as NO3- that promotes plant growth) CO Automobiles Burning wood Coal burning Plants CO air (changed to CO2 leads to global warming) trees & algae (absorbs it as CO2) oceans (absorbs more CO2 when the temp. is cooler) O3 sunlight + NO2 PAN NO2 PAN Aldehydees VOCs Aldehyde VOC’s Automobiles Paint Thinners Cleaning Solvents VOC’s air (as part of smog, many are carcinogenic) CFC’s Refrigerant Foam Cushions Cleaning Solvents CFC’s stratosphere (destroys O3) air (as smog, also eye irritant) air (eye irritant) air (eye & lung irritant) PM-10 Farming / Construction Burning wood or gas Industrial processes PM-10 air ( part of smog – causes respiratory distress especially in the presence of SO2) General Effects of Air Pollution Vegetation -- damage to leaf tissue, needles, or fruit (acid rain); clogging of stomata (particulates in smog); stunted growth (acidified soils); susceptibility to diseases or pests (tropospheric ozone, acidified soils); disruption of reproductive processes (acidified soils, ozone) Vertebrate Animals – impairment of respiratory system; damage to eyes (cataracts), teeth, and bones; increased susceptibility to disease; other stress-related environmental hazards; decreased availability of food sources; reduced ability to reproduce Humans -- toxic poisoning, causing cancer, birth defects, eye irritants, irritation of respiratory tract, increased susceptibility of viral infections, cause pneumonia and bronchitis, increased susceptibility to heart disease, aggravation of chronic diseases (asthma & emphysema) Soil & Water -- acidified; leaching of nutrients; releasing of toxic metals Man-made Structures – discoloration; erosion; decomposition of buildings, bridges & monuments Synergistic Effect – several pollutants have a more severe reaction when they are present at the same time than they do individually. Example: SO2 or NOx affix themselves to particulates in order to easily enter and stay lodged within the lungs. Without the particulates the reactions experienced from SO2 or NOx are not nearly so severe. 7 provisions of the Clean Air Act Establish NAAQS NAAQS (National Ambient Air Quality Standards) – 6 criteria pollutants (both primary and secondary) that monitor outdoor air on a daily basis (required by the Clean Air Act) – CO, SO2, NO2, PM-10, Pb, O3 Health Effects – CO, SO2, NO2, PM-10 (see Health Effects under Primary pollutants) O3 – eye, nose, throat irritant, aggravates respiratory conditions ( asthma, bronchitis, emphysema) Pb – causes mental retardation Prevent significant deterioration of air quality in areas where air quality is good Set national emission standards for toxic air pollutants for industrial emissions Require coal-burning power plants to cut SO2 emissions Require mobile sources to reduce NO2 emissions Require oil companies to sell cleaner burning gasoline Allow an Emissions Trading Policy (pollution credits) for SO 2 Factors increasing air pollution urban buildings – reduce wind speed hills and mountains – reduce air flow high temperatures - increase secondary air pollutants Thermal Inversions – when a layer of cool air is trapped beneath a layer of warm air which prevents the air currents from dispersing the pollutants Radiant – occurs at night when the ground cools faster than the air above it – is burned off by the sun Subsidence – when a warm weather front moves in over cooler air – may last from several hours to days Ways to REDUCE outdoor air pollutants (beyond sulfur reductions): Reduce PM-10 emissions by installing electrostatic precipitators, baghouse filters, or cyclone separators Change legislation to reduce particle size emissions to no more than PM-2.5 Use mass transit, bicycles, or walking instead of personal automobiles to reduce NO 2, hydrocarbons, & CO emissions Increase fuel efficiency of cars to reduce NO2, hydrocarbons, & CO emissions Provide for stricter emission controls of incinerators to reduce emissions of toxic compounds Provide tax credits for buying fuel efficient cars Use alternative fuels for cars (natural gas, ethanol, hydrogen fuel cells, electric –solar) Reduce the number of miles driven Retire older cars that pollute more Require stronger emissions inspections of all states Require emission standards for 2cycle engines (lawnmowers, leaf blowers, chain saws, outboard motors, sea and ski mobiles) Implementing policies to reduce CO2 and greenhouse gas emissions Indoor Air Pollution ***see your chart for indoor air pollutants, sources, health effects, and solutions. (or visit “The Toxic House” online for information)*** Additional indoor pollutants: Pollutant Source Asbestos pipe insulation, vinyl floor & ceiling tile Health Effects Lung disease (asbestosis), lung cancer Solutions Remove it from the premises Toxic Mold Respiratory distress, headaches Remove it from the premises Leaking pipes, condensation of air conditioners, water damage The 3 most dangerous indoor air pollutants: cigarette smoke, formaldehyde, radioactive radon-222 gas *** Review the handout on the effects that plants can have on indoor air pollution*** Sick Building Syndrome (SBS) – when an elevated level of indoor air pollutants makes the inhabitants sick (breathing problems, dizziness, eye, nose, throat irritation, sneezing, coughing, rash, sore throat, sinus problems) while they are in the building but their symptoms lessen or disappear when they are outside of the building.