Study Guide for Air Pollution Test
advertisement

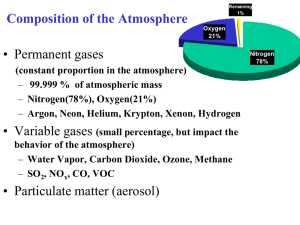
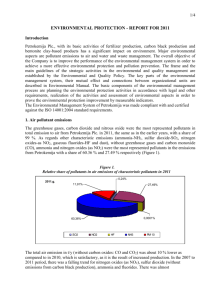
Study Guide for Air Pollution Test: Chapter 18 Review layers of atmosphere especially the roles of the troposphere and stratosphere Know factors that affect air pollution levels: climate (temperature and rainfall), thermal inversions. Be able to explain conditions that increase the likelihood of thermal inversions. Be able to define primary pollution and know the following examples: CO, CO2, SO2, NO, NO2, Volatile Organic Compounds (VOCs), and Particulate Matter (PM). Know the major sources (natural and anthropogenic) of these pollutants. Be able to define volatile organic compound (VOC) and know a few examples. Be sure to understand the difference between the air pollutant NO2 and the greenhouse gas N2O- see attached for review. Know that particulate matter is described in terms of size in micrometers (also known as microns which is represented by µm). PM 2.5 is particulate matter that is smaller than 2.5 µm. Know how the size of the particulate matter relates to its harmfulness to human health. Be able to define secondary pollution, photolysis and photochemical smog. Know the following examples of secondary pollutants: SO3, HNO3, H2SO4, O3, PAN’s. Be able to explain how temperature and time of day can affect levels of the secondary pollutants that are formed by photochemical reactions. Know the difference between gray-air (industrial) and brown-air (photochemical/NO2) smog. Know that at the high temperatures during combustion in automobile engines, coal-burning power plants and industrial plants can cause atmospheric nitrogen (N2) to react with O2 to produce nitric oxide N2 + O2 2NO Also understand the series of reactions of NO that can result in NO2 (in brown-air smog), O3 (groundlevel ozone pollution resulting from O released by photolysis if NO2 combining with O2), HNO3 (nitric acid – a cause of acid deposition) and PAN’s (peroxyacyl nitrates- from reactions with unburned hydrocarbons in the atmosphere). Understand the pH scale including the fact that it is logarithmic (base 10) so that each step on the pH scale is a 10 fold change in strength. Be able to define buffer and give examples of how acids can be buffered. Be able to define neutralization and name a substance that can be used to neutralize acidic lakes. Know the definition of acid deposition, including the pH to be defined as acid deposition. Know the chemicals found in acid deposition: HNO3, H2SO4 and H2CO3- (carbonic acid). Know the primary sources of these chemicals. Know some of the effects of acid deposition: Ecosystems: In addition to damaging organisms directly, acids can leach essential plant nutrients from the soil. Acid can mobilize toxic Al +3 ions. Acid conditions can cause moderately toxic inorganic mercury compounds to become highly toxic methylmercury. Building materials: Can damage building materials that contain calcium carbonate such as limestone and marble and can corrode metals. Be able to explain factors that make regions more vulnerable to acid deposition. Know strategies for preventing and cleaning-up acid rain. Know the effects of the following air pollutants: ozone (O3), carbon monoxide (CO), lead (Pb), particulate matter (PM), nitrogen dioxide (NO2) and sulfur dioxide (SO2). (See link on website for fact sheet http://www.arb.ca.gov/research/health/fs/fs2/fs2.htm ). Be able to explain why carbon monoxide is poisonous. Know the basics of the human respiratory system, and what the body’s natural defense mechanisms are. Know the basics of the Clean Air Act including the six pollutants that have criteria standards: NO2, SO2, carbon monoxide, lead, ozone and particulates. Know the most common indoor air pollutants and some sources. The four most dangerous are formaldehyde, Radon-222, asbestos, and smoke. Know factors that affect levels of indoor air pollutants (i.e. ventilation). Know methods of preventing or cleaning-up/diluting indoor air pollutants. Be able to define the sick building syndrome (when at least 20% of a building occupants suffer persistent symptom that disappear when they go outside.) Know methods to reduce air pollution: Best approach is to reduce causes (for example, by improved energy efficiency). Catalytic converters use catalysts that remove up to 90% of hydrocarbons, NOx and CO. Low sulfur coal techniques to clean coal (results in hazardous wastes that must be disposed) Unleaded gasoline Nitrous Oxide Vs. Nitrogen Oxides Nitrous Oxide: N2O (Laughing gas) Environmental Impact: Very strong greenhouse gas. Absorbs approximately 300 times more heat than CO2 (per molecule). Sources: Fertilizer use- Bacterial action on nitrogen compounds in fertilizers result in N2O production. High temperatures in vehicle engines and fuel-burning power plants causes N2 in the air to react with O2 to form N2O. Nitrogen oxides (NOx) Nitric oxide (NO) (NO and O2 form NO2 – so if NO also NO2) Nitrogen dioxide (NO2)- causes brown color of smog Environmental Impacts: Harmful to health- respiratory problems, impairs immune system. Reduces crop yields Results in several harmful secondary pollutants- see below Secondary Pollutants: NO2 reacts with oxygen and water vapor to form nitric acid (HNO3), a component in acid rain. A series of photolytic reactions of NO or NO2 with VOC’s can result in the formation of ground level ozone (a very harmful air pollutant) - Causes respiratory problems - Inhibits photosynthesis NOx’s can result in the formation of PAN’s (peroxylacyl nitrates) Sources: High temperatures in combustion engines in vehicles and burning of fuels in power plants results causes N2 in the air to react with oxygen to form NOx. Possible Reactions of Nitrogen Dioxide to Form Various Secondary Pollutants Hydrocarbons