raman
advertisement

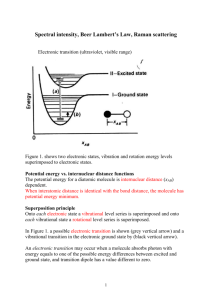
Raman scattering http://www.doitpoms.ac.uk/tlplib/raman/index.php http://www.chemistry.nmsu.edu/studntres/chem435/Lab15/ Raman scattering (sometimes called the Raman effect) is named after Indian physicist C. V. Raman who discovered it in 1928, though predictions had been made of such an inelastic scattering of light as far back as 1922. The importance of this discovery was recognised even then, and for his observation of this effect Raman was awarded the 1930 Nobel Prize in Physics. This was and remains the shortest time from a discovery to awarding of the Prize. In fact Raman was so confident that he arranged his travel to Stockholm several months in advance of the recipients being announced! This confidence seems quite justified, given that within a year and a half of his discovery, more than 150 papers mentioning the effect had been published. Since then Raman scattering has given rise to a number of important technologies, and foremost among these is Raman spectroscopy. Most light passing through a transparent substance undergoes Rayleigh scattering. This is an elastic effect, which means that the light does not gain or lose energy during the scattering. Therefore it stays at the same wavelength. The amount of scattering is strongly dependent on the wavelength, being proportional to l-4. (It is this fact that makes the sky blue, the shorter wavelength blue components in the Sun’s light are Rayleigh scattered in the atmosphere far more than the longer wavelengths. Blue light is then seen coming from all over the sky. The scattering of blue light from its direct path from the Sun also causes the Sun itself to appear yellow.) In Rayleigh scattering a photon interacts with a molecule, polarising the electron cloud and raising it to a “virtual” energy state. This is extremely short lived (on the order of 1014 seconds) and the molecule soon drops back down to its ground state, releasing a photon. This can be released in any direction, resulting in scattering. However since the molecule is dropping back to the same state it started in, the energy released in the photon must be the same as the energy from the initial photon. Therefore the scattered light has the same wavelength. Raman scattering is different in that it is inelastic. The light photons lose or gain energy during the scattering process, and therefore increase or decrease in wavelength respectively. If the molecule is promoted from a ground to a virtual state and then drops back down to a (higher energy) vibrational state then the scattered photon has less energy than the incident photon, and therefore a longer wavelength. This is called Stokes scattering. If the molecule is in a vibrational state to begin with and after scattering is in its ground state then the scattered photon has more energy, and therefore a shorter wavelength. This is called anti-Stokes scattering. Three different forms of scattering Only about 1 in 107 photons undergo Stokes Raman scattering and so this is usually swamped by the far more prominent Rayleigh scattering. The amount of anti-Stokes scattering is even less than this (see comment 1 for an explanation). The shift due to the Raman effect is determined by the spacing between the vibrational states and the ground states i.e. by the phonons of the system. The Stokes and anti-Stokes scattered light will be shifted an equal distance on opposite sides of the Rayleigh scattered light. Therefore the spectrum is symmetrical about the wavelength of light used, apart from the difference in intensities. Normally in Raman spectroscopy only the Stokes half of the spectrum is used, due to its greater intensity. In one of Raman’s experiments demonstrating inelastic scattering he used light from the Sun focused using a telescope to obtain a high intensity light. This was passed through a monochromatic filter, and then through a variety of liquids where it underwent scattering. After passing through these he observed it with a crossed filter that blocked the monochromatic light. Some light was seen passing through this filter, which showed that its wavelength had been changed. Comment 1. Stokes and anti-Stokes scattering back The difference in intensity of the Stokes and anti-Stokes components is due to the different number of molecules in each state initially. The population follows the Boltzmann distribution: Therefore there are exponentially fewer molecules that start out in the higher energy vibrational state. Since it is these that give rise to the anti-Stokes scattering, this is much less intense. The disparity depends on the spacing of the energy levels. So for the less widely spaced rotational levels, the Stokes and anti-Stokes scattering are of similar magnitude. For the vibrational levels, which are spaced further apart, the anti-Stokes signal is significantly weaker than the Stokes signal. The disparity is also reduced with increased temperature, and the difference can be used as a measure of temperature. Comment 2 Vibrational states The frequencies of a molecule’s vibrational modes, and hence the shifts in wavenumber, are determined by the reduced mass μ and the bond strength k. As a simple example N2 has only one vibrational mode. The reduced mass is given by: The two nitrogen atoms are joined by a strong triple bond, with a force constant k = 22.95 N cm-1. The vibrations are simple harmonic, so the frequency, and hence wavenumber, can be determined: In this example the wavenumber is 23590 m-1 or 235.9 cm-1. Comment