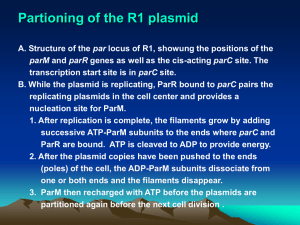
Supplementary Materials and methods (doc 154K)
advertisement

MATERIALS AND METHODS Bacterial strains, plasmids and antibiotic susceptibility testing. The bacterial strains and plasmids used in this study are detailed in Table 1. P. aeruginosa PAO1 was transformed by electroporation with the different plasmids as 5 previously described (Choi and Schweizer 2006) using a Gene Pulser apparatus (BioRad). Transformants were selected on LB agar plates containing antibiotics. The antibiotics and concentration used for the different plasmids were: pNI105, neomycin 50 μg/ml; pBS228, tetracycline 25 μg/ml; Rms149, streptomycin 200 μg/ml; pAKD1, streptomycin 200 μg/ml; PAMBL-1, gentamycin 32 μg/ml; PAMBL-2, streptomycin 10 200 μg/ml. Plasmid transformation was confirmed by PCR using GoTaq green Mastermix (Promega, Madison, WI, USA) (Table S2) and by plasmid extraction using QIAprep Spin Miniprep (Qiagen, Inc., Chatworth, California, USA). The antibiotic resistance profiles of the different strains used in this study are detailed in Table S1 and Figure S1. Minimal inhibitory concentrations (MIC) were performed in triplicate 15 following Clinical and Laboratory Standards Institute guidelines (2009). Briefly, an initial concentration of 5x105 CFU/ml of the different PAO1 strains was challenged to grow in Mueller-Hinton broth (Fisher Scientific, NJ, USA) with doubling concentrations of the antibiotics. Bacteria were incubated overnight for 20 hours. The MIC was determined as the lowest concentration of antibiotic inhibiting bacterial 20 growth. Antibiotics were supplied by Sigma-Aldrich (Sigma Chemical Co. St. Louis, MO, USA). pNI105 mobilization experiments The mobilization of plasmid pNI105 (conferring resistance to kanamycin and neomycin) by the conjugative plasmids pAKD1, PAMBL-1 and PAMBL-2 was 25 assessed. We used the protocol previously described by Itoh et al. for the mobilization of plasmid pNI111 by RP4 (Itoh et al 1991). P. aeruginosa PAO1-GFP (resistant to gentamycin and chloramphenicol) was used as a recipient strain. Mating experiments were performed three independent times on LB agar (Fisher Scientific, NJ, USA) at 37ºC. Transconjugants of P. aeruginosa PAO1-GFP carrying pNI105 were selected 30 on LB agar containing the appropriate antibiotics (neomycin 50 mg/L, kanamycin 1200 mg/L, gentamycin 128 mg/L and chloramphenicol 50 mg/L), donors were selected on LB agar with neomycin (50 mg/L) and kanamycin (1200 mg/L). Competitive fitness assays The fitness of each strain carrying the different plasmid combinations was determined 35 relative to the PAO1-GFP strain. Pre-cultures of the strains were incubated at 37ºC with 200 RPM shaking overnight in 3 mL of LB broth (Fisher Scientific, NJ, USA). Pre-cultures were diluted 100-fold in 3 ml of fresh LB and incubated in the same conditions until they reach mid-exponential phase (OD600 of ≈ 0.5). Cultures of the strains were then mixed at a ratio of approximately 50% PAO1 carrying plasmid to 40 50% PAO1-GFP. Competitions starting at 50% of each strain maximize the ability to detect small differences in fitness. The exact initial proportions were confirmed via flow cytometry (see conditions below). Mixtures were diluted 200-fold in fresh LB and competed for 16 hours at 37°C with no agitation (~7 generations). Again, the final proportion was measured by flow cytometry. The fitness of the strain carrying the 45 plasmid(s) relative to the PAO1-GFP strain was determined using the formula: W plasmid æN ö log e ç final, plasmid ÷ è N initial, plasmid ø = æN ö log e ç final,GFP ÷ èN ø initial,GFP Where wplasmid is the fitness of the strain carrying the plasmid, Ninitial, plasmid and Nfinal, plasmid are the numbers of cells of the strain carrying the plasmid before and after the competition, and Ninitial, GFP and Nfinal, GFP are the numbers of cells of PAO1-GFP 50 before and after the competition. The fitness of each strain was calculated using 3 independent competition experiments with 5 technical replicates per experiment. In every assay, wild type PAO1 was competed with PAO1-GFP to set the discriminatory gates for the flow cytometer and to calculate the results of the competitions relative to the parental PAO1 strain. The average fitness of PAO1-GFP relative to PAO1 was 55 0.977 (SE= 0.011, 3 biological replicates of 5 technical replicates each). To confirm the results of the competition experiments we competed all the strains carrying pairs of plasmids against PAO1-GFP-tagged strain carrying plasmid pNI105 (data not shown). Fitness values obtained in these competitions were similar to those obtains using PAO1-GFP (R2= 0.907) (Pearson's test, corr= 0. 0.952, P= 0. 012, t= 5.41, 60 df=3). Flow cytometry was performed using an Accuri C6 Flow Cytometer Instrument (BD Accuri, San Jose, CA, USA) under the following parameters: flow rate: 66 uL/min, core size: 22 um, total number of events recorded per sample: 10,000. To detect GFP fluorescence samples were measured using the FL1 channel, filter range: 533 nm (+/- 15). Bacteria cell size was not affected by plasmid carriage. Cell 65 size was measured using the average forward scatter value of monocultures of the strains carrying the different plasmids during flow cytometry, and controlled using confocal microscopy. One possible factor that would have altered the result of the competitions would be plasmid transfer by conjugation from PAO1 to PAO1-GFP. If plasmids were transferred to PAO1-GFP they would produce a fitness cost, altering 70 the competition outcome. To control for plasmid transfer by conjugation during the competition experiments, conjugation experiments between the plasmid-carrying PAO1 and a PAO1-GFP were performed in the exact same conditions as the competitions (in triplicate). Aliquots of the conjugation mix were plated on LB containing the appropriate antibiotics to select for the transconjugants and the donors. 75 The conjugation frequencies for all the plasmids and strains were <10-6 transconjugants per donor colony forming unit. Hence, conjugation did not affect the results of the competitions assays. Quantification of plasmid copy numbers. The copy numbers of plasmids were determined by quantitative polymerase chain 80 reaction (qPCR) using an ABI Prism 7000 sequence detection system (Life Technologies, USA). DNA extraction, quantification and digestion. To analyse the plasmid copy numbers in the different constructions, three to six independent DNA extractions were done per strain and qPCR was performed for each extraction in triplicate. DNA extractions 85 were performed from 1 ml of LB broth cultures without antibiotics prepared as for the competitive fitness assays (above) and using a Qiagen DNeasy Blood and Tissue Kit (Qiagen, Inc., Chatworth, California, USA). DNA was quantified using the QuantiFluor dsDNA system (Promega, Madison, WI, USA), following manufacturers’ instructions. Restriction-enzyme digested total DNA is a better 90 template source than non-digested total DNA for plasmid quantification by qPCR (Providenti et al 2006). Linearizing the plasmids increases accessibility to plasmid DNA template, preventing copy number from being underestimated (Providenti et al 2006). Therefore, 1 μg of DNA from each sample was digested using 20 units of EcoRI (Fermentas, Thermo Fisher Scienific, USA) for 2 hours at 37ºC. EcoRI was 95 inactivated at 65ºC for 20 min. No EcoRI targets are present in the amplification product of any of the reactions. qPCR. We developed a specific qPCR for each plasmid and we used a previously described qPCR of the rpoD chromosomal monocopy gene to compare the ratio of plasmid and chromosomal DNA (Savli et al 2003). In Table S2 we present the 100 primers and conditions used for this analysis, as well as the efficiency of the reactions and the target and size of the amplicon produced (~180-190 bp). Efficiency of the reactions was calculated from the standard curve generated by performing qPCR with 4 5-fold dilutions of template DNAs in triplicate (~2 ng/μl to 16 pg/μl working range of DNA concentration). qPCRs were performed using a SensiMix SYBR Hi-ROX Kit 105 (Bioline, UK) at a final DNA concentraction of 0.2 ng/μl. The amplification conditions were: initial denaturation for 10 min at 94°C, followed by 30 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 55-60°C (Table S2) and extension for 30 s at 72°C. Inter-run calibration samples were used to normalize the results from different plates of each qPCR. 110 Calculation of plasmid copies. To calculate the copy number of plasmid per chromosome we used the formula: Ctc 1+ Ec ) ( S cn = * c (1+ E ) p Ctp Sp Where cn is the plasmid copy number per chromosome, Sc and Sp are the sizes of the chromosomal and plasmid amplicons (in bp), Ec and Ep are the efficiencies of the 115 chromosomal and plasmid qPCRs (relative to 1), and Ctc and Ctp are the threshold cycles of the chromosomal and plasmid reactions, respectively. Plasmid stability determination. We assessed the stability of plasmids after 100 generations of propagation in antibiotic-free medium. Six lines of each strain were cultured in LB broth. Every day, 120 the six lines of each strain were diluted 1000-fold into 2 mL of fresh LB medium without antibiotics. Cultures were incubated at 37ºC with 225 RPM shaking. Lines were evolved for 10 days (~100 generations). On day 10, samples were plated on LB agar without antibiotics and 50 colonies of each line were replicated on media containing antibiotic. The strains with one plasmid were replicated on one plate and 125 the strains with two plasmids were replicated on two plates, each plate containing the selective antibiotic for the specific plasmid being assayed. The antibiotics and concentration used for the different plasmid bearing strains were the same used for the plasmid transformation: pNI105, neomycin 50 μg/ml; pBS228, tetracycline 25 μg/ml; Rms149, streptomycin 200 μg/ml; pAKD1, streptomycin 200 μg/ml; PAMBL-1, 130 gentamycin 32 μg/ml; PAMBL-2, streptomycin 200 μg/ml. To control for the quality of the different plates with antibiotics, colonies of PAO1 and PAO1 with all the plasmid combinations were independently replicated to check that the plates inhibited those strains without the appropriate plasmids. The proportion of bacteria harbouring plasmids was determined by counting the number of replicates growing on antibiotic- 135 containing medium. In addition, for one of the lines of each strain, 20 of the colonies that were replicated were also tested by standard PCR to confirm the presence/absence of plasmid (Table S2). The PCR results were the same as those of the phenotypic assay in every case. In addition, PCR mapping of pNI105 was performed from the DNA extraction of every line containing it after the 100 140 generations of evolution, confirming that no major genetic rearrangement which may have affected its stability had taken place. pNI105 PCR mapping was performed using pNI105-F/pNI105map-R and pNI105-R/pNI105map-F primers pairs, producing 3.7 and 1.8 kb amplification products, respectively (Table S2). PCRs were performed using GoTaq green Mastermix (Promega, Madison, WI, USA) and following 145 manufacturers’ instructions. Bioinformatics and plasmid distribution analysis. We analysed all the bacterial genomes available in the GenBank database (National Center for Biotechnology Information, NCBI) as of October 2012. Only the genomes with the status of “Chromosomes” were included in the analysis, and those under the 150 status of “Scaffolds or contigs”, “SRA or Traces” or “No data” were excluded since they are not properly assembled and plasmids are not annotated. We scanned 1866 genomes of free-living bacteria from the 31 different eubacteria phyla in the NCBI database. Endosymbiont species and obligate intracellular parasites were excluded due to their extremely reduced chromosomal and plasmid sizes. Members of the 155 genus Borrelia were also excluded due to their highly unusual genomes, which include a linear chromosome and as many as 20 linear or circular plasmids (Chaconas and Kobryn 2010). The genomes were examined for the presence of plasmids, and both the number of plasmids and their sizes were analysed. To study small-large plasmid associations, the limit size between small and large plasmids was calculated 160 independently for Enterobacteriaceae, Bacillaceae and Staphylococcaceae. Threshold values were determined by visually inspecting histograms of plasmid size for a region near the geometric mean which displays a lack of data. The cut-off was defined as the geometric mean of the two plasmid sizes flanking this region (Figure 5A). The expected frequencies of strains with different plasmid associations was calculated 165 from the actual number of strains per category (carrying 0, 1, 2, 3, 4… plasmids) in each group but using the overall frequency of small and large plasmids of the group. The expected frequencies of small and large plasmids within categories were calculated for each family under the null hypothesis that associations between large and small plasmids are random, using the actual number of plasmids per category and 170 the overall frequency of small and large plasmids of the group. To check the general tendency towards plasmid coexistence, the expected distribution of genomes carrying different numbers of plasmids was calculated following a Poisson distribution (using the specific average of plasmid per strain observed in the different groups analysed), and then compared to the observed frequencies. 175