The discovery of new elements
advertisement

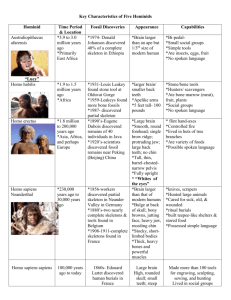
The discovery of new elements How are new elements discovered? There are probably nearly as many answers to this question as there are elements. Many elements were found more or less by accident. Others were discovered as a result of research into a particular compound or mineral. Others were predicted to exist – on the basis of Mendeleev’s Periodic Table, for example – so the discoverer knew what he or she was looking for. However, from time to time a new chemical technique is developed or discovered that leads to the discovery of several new elements in a short time. You can use the interactive Periodic Table to investigate this idea. Activity The following activities are based around the interactive Periodic Table. a) Run the Animate function and watch as the elements are displayed in the order in which they were discovered over time. Do they seem to appear at a steady rate? b) Use the Histogram function to plot the number of elements discovered in each century. Does this confirm your impression? You should see that there are several periods in which many elements were discovered with periods of time in between when none were discovered. Some of the bursts of discovery were caused by the development of new chemical techniques. It is a great achievement to discover a single element out of the 111 or so that are now known. So you may be surprised to find that there are several people who have discovered more than one element, in one case as many as 11. Here are some brief descriptions of the work of some chemists who discovered more than one element. In many cases they took advantage of a new technique. Jons Jacob Berzelius – reaction with carbon Humphry Davy – electrolysis Robert Bunsen – flame colours William Ramsay – the distillation of liquid air Marie Curie - radioactive elements Glenn T Seaborg – making new elements with sub-atomic particles Jons Jacob Berzelius – reaction with carbon Jons Jacob Berzelius (see box) obtained four elements (thorium, cerium, selenium and impure silicon) mainly by reduction with carbon. Activity a) Ytterby has the unique distinction of having four elements named after it. Look at the Periodic Table and suggest what these elements might be. You could try to confirm your suggestions by doing a search on the internet or research in a library. b) Use an internet search engine to help you find out what sort of symbols were used by chemists to represent elements before the letter symbols introduced by Berzelius in the early 1800s. Jons Jacob Berzelius Jons Jacob Berzelius (1779 - 1848) is probably Sweden's most famous chemist. As well as his own discoveries, some of his colleagues also discovered elements. Lithium was discovered by Johann Arfvedson, whom he had trained, and Berzelius’ former pupil in Stockholm (Carl Mosander) discovered lanthanum, erbium and terbium. Mosander did not use a new technique but was fortunate to work near Ytterby - a village near Vaxhol, Stockholm in Sweden with a quarry where compounds containing many of the elements of atomic numbers between 58 and 71 (called the lanthanides) were found. Berzelius was also the first to use letter symbols for elements like the ones we use today. Jons Jacob Berzelius. Reproduced courtesy of the Library and Information Centre, The Royal Society of Chemistry. Question Q 1. Berzelius used the technique of reaction with carbon to isolate elements from their compounds. One common compound of silicon is silicon dioxide (SiO 2), which is found in sand. (a) Suggest word and symbol equations for the reaction of carbon with silicon dioxide to form silicon. (b) What other compound would be produced? Humphry Davy - electrolysis Humphry Davy (see box) took advantage of new technology to discover six elements. He used the then-newly invented voltaic pile (we would call it a battery), to pass electricity through molten salts of alkali metals. This produced highly reactive metals at the negative electrode. He obtained sodium and potassium, then magnesium, calcium, barium and strontium. Sir Humphry Davy Davy (1778 - 1829) was born Cornwall but worked in Bristol and then at the Royal Institution in London which is still a prestigious centre of scientific research. Like many scientists at the time Davy worked in many fields. He investigated the anaesthetic properties of dinitrogen oxide (‘laughing gas’) on himself, and his name will always be associated with the miners’ safety lamp which he invented to prevent explosions in mines caused by the use of naked flames. He was One of Davy’s lectures at The Royal Institution. Reproduced courtesy of the Library and Information Centre, The Royal Society of Chemistry. well-known as a lecturer and was succeeded by his assistant, Michael Faraday, who became equally famous as a scientist for developing the electric motor and generator. Questions Q 2. (a) Why could Davy not isolate, say, sodium from sodium oxide by reducing the oxide with carbon as Berzelius had done with silicon, cerium, thorium and selenium? (b) Why did Davy electrolyse molten salts rather than solids? (c) Why did Davy electrolyse molten salts rather than solutions of salts in water? Robert Bunsen - flame colours Robert Bunsen (1811 - 1899) and Gustav Kirchoff (1824 - 1887) (see box) were early users of the technique of examining the light given out by heated compounds to recognise new elements. Have you noticed that a wire dipped into sodium chloride solution gives an intense yellow flame colour or that when a pan of salted water boils over it colours the gas cooker flame yellow? This colour is characteristic of sodium and is also seen in street lamps that are filled with sodium vapour. A development of this method is still used today to measure the amounts of certain elements in mixtures. Robert Bunsen and Gustav Kirchoff In 1861 Bunsen and Kirchoff jointly discovered caesium (which gave a blue flame) and rubidium (which gave a red flame). Bunsen (who devised, or at least developed, the Bunsen burner) discovered only two elements himself, along with Kirchoff, but his technique was used to discover several more. Paul Emile Lecoq de Boisbaudran (1838 - 1912) used flame colours (called emission spectra) to search for more elements. He discovered gallium (1875), samarium and dysprosium. Gallium was the first element to be found whose properties matched elements predicted in detail by Mendeleev in 1870, dramatic proof of his ideas about the Periodic Table. Kirchoff (left) and Bunsen. Reproduced courtesy of the Library and Information Centre, The Royal Society of Chemistry. Activity a) The flame colours of some metals are clear enough to be used to identify them by eye – the orange-yellow colour of sodium is an example. Make a list of flame colours of metals that you know, then use an internet search to check your answers and fill in some you are not familiar with. b) The element helium was discovered from its spectrum. Find out using an internet search what was unusual about this discovery. William Ramsay - the distillation of liquid air William Ramsay (see box) investigated the observation that nitrogen made by removal of other gases from air had a slightly different density to nitrogen made by chemical decomposition. First he discovered argon and then predicted a complete family of elements between Groups 7 and 1 of the Periodic Table. We now call these the noble gases. By fractional distillation of liquefied air, he and Morris Travers then discovered neon, krypton and xenon. Ramsay won the 1904 Nobel prize for chemistry. When Ramsay discovered a gas of relative mass approximately 40, chemists were at first reluctant to believe that a whole new group of elements remained to be discovered. At first, Mendeleev was a disbeliever because he felt that the discovery undermined his Periodic Table. Later, however, he came to see that it was actually a confirmation of the basic idea behind the Table. Some tried to explain the new gas as an allotrope of nitrogen, N3. (Allotropes are forms of the same element which differ in the arrangement of their atoms.) This seemed possible because oxygen has an allotrope O3, usually called ozone. N3 would have a relative molecular mass of 42, close to the measured value for argon of 40. However, N3 has, up to now, never been found or made. Sir William Ramsay Ramsay (1852 - 1916) was Glasgow-born but his main research was at London University. He discovered argon by taking a sample of air and first removing all the oxygen. He then passed the remaining gas (mostly nitrogen) over hot magnesium. Magnesium is reactive enough to combine with nitrogen to leave a solid called magnesium nitride. After doing this repeatedly, he was still left with some gas whose relative mass was 40. This gas didn’t seem to fit into Mendeleev’s Periodic Table! Later, using newly-discovered techniques for cooling and liquefying gases, he was able to separate other gases from air – neon, krypton, xenon and radon – and it was realised that he had discovered a whole group of elements, none of which had been known to Mendeleev. The final member of the group, helium, had been discovered a few years earlier – in the Sun. Pierre Jules César Janssen had noticed some lines in the spectrum of sunlight that didn’t belong to any known element and suggested a new element, which he called helium, existed in the Sun. Ramsay was the first to recognise helium on Earth so he discovered all of the noble gases (almost). Sir William Ramsay. Reproduced courtesy of the Library and information Centre, The Royal Society of Chemistry. Questions Q 3. (a) One possible way of removing oxygen from the air is to pass it over heated copper. Write a word equation for the chemical reaction that happens. (b) Why will copper react with oxygen but will not react with nitrogen? (c) Why will magnesium react with nitrogen? Write a word equation for the chemical reaction that happens. (d) What other gases would Ramsay have had to remove from air before he could start his experiments? Suggest a way of removing each of these gases. Marie Curie - radioactive elements Marie Curie, along with her husband, Pierre (see box), investigated radioactive elements, eventually extracting less than a gram of a new element, radium, from over eight tonnes of the ore pitchblende. Marie Curie Marie Curie (1867 - 1934) discovered two elements as she investigated what became known as radioactivity. First she identified polonium, which she named after her native Poland, then, with her husband Pierre, she found the more intensely radioactive element radium. She won the 1911 Nobel prize for chemistry for discovering the two elements after she had shared the 1903 physics prize with Pierre, and Henri Becquerel. She is unique in being a double Nobel prize winner, having a chemical element (curium) and a unit (the curie, which measures radioactivity) named after her. Marie Curie and husband Pierre. Reproduced Courtesy of the Library and Information Centre, The Royal Society of Chemistry. Activity a) Search the internet to find lists of Nobel prize winners in science (there are three science categories – Chemistry, Physics, and Physiology and Medicine) to find out what else is unique about the Curie family and the Nobel prize. b) Search the internet to find which other elements were discovered by women. Glenn T Seaborg - making new elements with sub-atomic particles Glenn T Seaborg (see box), with his co-workers identified 11 elements. These all have atomic number greater than 92 (uranium) and are man-made rather than occurring naturally. The discovery that uranium atoms could be bombarded with neutrons to create new elements led to an extension of the Periodic Table beyond uranium. The elements found by chemists before Seaborg were discovered – they existed on Earth already, combined with other elements to form compounds in most cases. Chemists had to extract them and show that they really were new elements. The elements found by Seaborg and his colleagues were actually made – they are elements that do not exist naturally on Earth. The heaviest element that does exist on Earth is uranium which has 92 protons. Protons are positively charged and tend to repel one another (they are held in the nucleus against this repulsion by a force called the strong nuclear force). Atoms whose nuclei have more than 92 protons tend to break apart because of this repulsion. This is why they are not found on Earth. Scientists found that when they fired neutrons at uranium atoms, one would occasionally stick to a uranium nucleus. This increased the relative atomic mass of the atom by one but kept the atomic number the same. Sometimes this neutron then ‘spat out’ an electron and turned into a proton. This meant that the nucleus now had 93 protons and was a new element, of atomic number 93, which was christened neptunium, Np. This was actually done by Edwin McMillan and Philip Abelson. Seaborg then took over working as the leader of a group of scientists. Bombardment with other sub-atomic particles allowed them to make elements numbers 94 - 103 in the same sort of way. Glenn T Seaborg Seaborg (1912 – 1999) won the 1951 Nobel prize for chemistry. After some argument between the USA and the rest of the world, element 106 was named seaborgium shortly before he died. This was a matter of some controversy because the International Union of Pure and Applied Chemistry, IUPAC, the body that deals with naming in chemistry, had previously ruled that elements should not be named after living people. Glenn T Seaborg (left) with United States president John F Kennedy. Reproduced Courtesy of the Ernest Orlando Lawrence Berkeley National Laboratory. The elements of atomic number greater than 92 are all radioactive; their nuclei break up changing them into different elements. In some cases, this can happen only fractions of a second after they have been made, making it difficult to be sure that they have actually been made. We say they have very short half lives. There are only three research centres that are capable of making these so-called transuranic elements, one in Berkeley, California in the USA, one in Dubna in Russia and the third in Darmstadt in the former East Germany. Often, two centres claimed to have made a particular element first and therefore also claimed the right to name it. A situation arose where several elements had more than one name. So, to avoid confusion, IUPAC made a temporary ruling that the elements from atomic number 104 onwards should be named unnilquadium, unnilpentium etc from the Latin for 104, 105 and so on. Later IUPAC examined the evidence and decided which research team had actually discovered which element and allowed the discoverers to name them. More controversy followed and IUPAC changed its ruling which led to further changes. So the same name was sometimes used for two different elements at different times! The situation is summarised in the Table below. Atomic number 104 105 106 107 108 109 Initially suggested name Rutherfordium, Hahnium, Hn or Seaborgium, Sg Rf or Nielsbohrium, Kurchatovium, Ns Ku Nielsbohrium, Ns Hassium, Hs Meitnerium, Mt IUPAC temporary name Unnilquadium, Unq Unnilpentium, Unp Unnilhexium, Unh Unnilseptium, Uns Unniloctium, Uno Unnilennium, Unn Agreed name 1995 Dubnium, Db Joliotium, Jl Rutherfordium, Rf Bohrium, Bh Hahnium, Hn Meitnerium, Mt Agreed name 1996 Rutherfordium, Rf Dubnium, Db Seaborgium, Sg Bohrium, Bh Hassium, Hs Meitnerium, Mt Activity Most, but not all, of the names of elements 104 - 109 honour scientists such as Seaborg. Hassium is named after Hesse, the province where the German research centre is located and Dubnium after the location of the Russian research centre. Use an internet search to find out who the other elements are named after and what their scientific achievements were. Which scientist would you suggest is worthy of having a new element named after him or her? Answers to questions Q 1. (a) silicon dioxide + carbon → silicon + carbon dioxide SiO2(s) + C(s) → Si(s) + CO2(g) (b) The new compound is carbon dioxide Q 2. (a) Carbon is not reactive enough to remove oxygen from sodium oxide. (b) Solid salts do not conduct electricity, while molten ones do (because the ions are free to move in liquids but not in solids). (c) Any sodium produced would immediately react with water / the products of the electrolysis of water would also be formed. Q 3. (a) copper + oxygen → copper oxide (b) Nitrogen is much less reactive than oxygen. (c) Magnesium is one of the more reactive elements. magnesium + nitrogen → magnesium nitride Note the name of the product is magnesium nitride, not magnesium nitrate. The ending -ide means that there are only two elements in the compound. The ending -ate means that there are at least three elements, one of which is oxygen. (d) Carbon dioxide and water vapour. Carbon dioxide (an acidic gas) could be removed by passing air over an alkali and water by passing it over a drying agent such as anhydrous copper sulfate.