Determining Chemical Formulas
advertisement

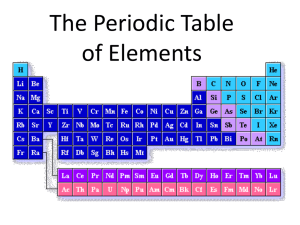
Determining Chemical Formulas A Yakima WATERS Mini Lesson Targets and Assessment WA Science Standards Addressed: 9-11 PS2C When elements are listed in order according to the number of protons, repeating patterns of physical and chemical properties identify families of elements with similar properties. This Periodic Table is a consequence of the repeating pattern of outermost electrons. Assessments: Formative assessment: pre-exercise quiz (see attached. Summative assessment: post exercise quiz (see attached) Lesson Parameters Content Area: Chemistry Overview: Students will be using paper models of elements to make chemical bonds which they will then use to write chemical formulas. Grade Level: 10, 11, 12 Suggested Time: 1.5 to 2 hours Special Materials: Paper models of elements (see accompanying document) Learning Outcomes: Knowledge: Upon completion of this lesson, the students should be able explain the basic principles of chemical bonding and the concept of chemical formulas using the following terms: o Ionic bond o Covalent bond o Valence electrons o Octet rule o Ionization Skill: Students should be able to correctly write chemical formulas and explain the concepts behind chemical bonding. Science Concept Background: A chemical formula is a combination of elemental symbols and numbers used to show the composition of a compound made by chemical bonding. The number of valence electrons, types of bonding, and special characteristics must be taken into account. The number of valence electrons, or electrons found in the outer shell of an atom, is very important when figuring out what the element will bond with. Elements generally want to fill their outer subshell to comply with the Octet Rule which makes them more stable. The number of valence electrons can be figured out by using the periodic table. Any element in group 1 (running vertically) will have 1 valence electron. Elements in group 2 will have two valence electrons, and so forth through the rest of the eight groups in the periodic table. The only exception to this rule is found with the transition metals, why lie in the middle of the periodic table. They can be found with a different number of valence electrons depending on what state or environment they are in. When two elements share valence electrons to fulfill the octet rule, they form a bond (either covalent or ionic). An ionic bond consists of two elements- one being a metal (including the transition metals) and one being a non-metal. A covalent bond, however, is formed between two non-metal elements. It is important to distinguish between the two when it comes to figure out the chemical name, but for figuring out chemical formulas it’s not very necessary. There are some special cases where these rules don’t necessarily apply, as with acids and hydrates. Acids have their own formula (usually starting with Hydrogen) and they usually have a common name and hydrates are usually found in the presence of water. For the purpose of this lab, both groups are not included. However it could be expanded to include them. Materials: Scissors, glue, pencil, provided worksheets (see attached) Procedure: 1) Give the formative assessment provided (see attached) the day prior to the lesson. This will help the instructor have an idea of what your students understand about the periodic table and chemical formulas. 2) Give a lecture on the periodic table: a. Groups of the periodic table (including valance electrons) b. Octet rule c. Introduce chemical formulas. 3) Hand out exercise and have them complete it (following directions provided). 4) Follow up: have students group in two and present each answer to the class; explaining why how they came about their answer. 5) Follow up with summative assessment. Extension(s): This lesson is useful because it not only teaches the students about writing chemical formulas, but it also leads into different types of chemical bonding. A lesson on chemical bonding and naming would great to come after this exercise Teaching Tips: For this lesson it is important for the students to have some background knowledge of the periodic table. They should be able to calculate the number of valence electrons based on the group number and predict what elements they would potentially bond with to fulfill the octet rule. Use the pre-exercise quiz to gauge where to start your lecture based on this assignment. Supplements: Chemistry text book (which ever your class uses), periodic table, helpful video: www.youtube.com/watch?v=vscoYh6m46M, helpful website for additional assistance: www.chemicalformula.org/chemistry-help/writing-chemical-formula By: John (Eric) Inions, Fall 2011, for Davis High School Determining Chemical Formulas Name:_____________ Directions: Cut out the ions from the two attached pages as needed. For questions 1-5: glue them in place, and write the formula for each. Follow your teacher’s example. Then write the formulas for the rest using the ion cut-outs for help. 1. Magnesium Chloride Formula: 2. Hydrogen Iodide Formula: 3. Potassium Sulfide Formula: 4. Lithium Carbonate Formula: 5. Hydrogen Fluoride Formula: 6. Calcium Hydroxide 16. Rubidium Iodide 7. Copper (II) Oxide 17. Sodium Chloride 8. Iron (II) Chloride 18. Radium Bromide 9.Aluminum Sulfate 19. Zinc Oxide 10. Aluminum Oxide 20. Calcium Oxide 11. Calcium Nitrate 21. Aluminum Bromide 12. Sodium Cyanide 22. Barium Cyanate 13. Copper (I) Sulfate 23. Sodium Thiosulfate 14. Ammonium Hydroxide 24. Ammonium Phosphate 15. Calcium Hypochlorite 25.Lithium Fluoride Pre-exercise Quiz Name:________________ 1. Describe the following terms (trends) from the periodic table. Use examples if necessary. a. Familiesb. Groupsc. Transition metals- 2. Define valence electrons: 3. Define and describe the Octet Rule. Use examples if necessary. Post exercise Quiz Name:________________ 1. Define and describe the Octet Rule. Use examples if necessary 2. Why do elements (atoms) want to bond with others? 3. Determine the chemical formulas for the following compounds a. Calcium Oxide b. Aluminum Oxide c. Barium Cyanide d. Zinc Oxide e. Copper (II) Sulfate Answer Key: Pre-Lesson 1. A. 2. 3. Families- are groups of elements that have similar chemical properties. Examples: alkali metals, Halogen gases, Noble gases, etc. B. Groups- separated into vertical columns. All the elements in the same group have the same number of valence electrons. C. Transition metals- don’t follow the octet rule. They can have more than eight electrons in their shell. Valence electrons are the electrons in the outer shell of an atom. They are the ones responsible for reacting with other electrons forming chemical bonds. The octet rule says that atoms prefer to have a stable outer shell having composed of eight electrons. Atoms will tend to give up or receive electrons to fill their outer shell. Answer Key: Post-Lesson 1. 2. 3. The octet rule says that atoms prefer to have a stable outer shell having composed of eight electrons. Atoms will tend to give up or receive electrons to fill their outer shell. Atoms want to bond with another in order to become more stable fulfilling the octet rule. a. CaO b. Al2O3 c. Ba(CN)2 d. ZnO e. CuSO4