CHEMISTRY 161 - Seattle Central College
advertisement
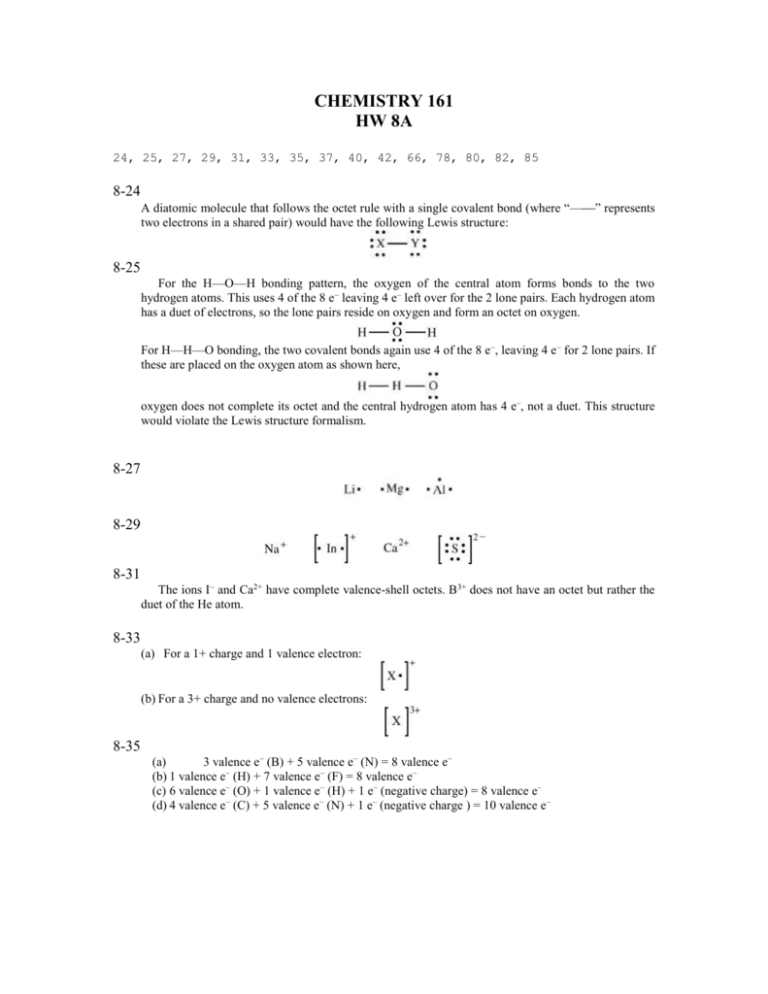
CHEMISTRY 161 HW 8A 24, 25, 27, 29, 31, 33, 35, 37, 40, 42, 66, 78, 80, 82, 85 8-24 A diatomic molecule that follows the octet rule with a single covalent bond (where “—” represents two electrons in a shared pair) would have the following Lewis structure: 8-25 For the H—O—H bonding pattern, the oxygen of the central atom forms bonds to the two hydrogen atoms. This uses 4 of the 8 e– leaving 4 e– left over for the 2 lone pairs. Each hydrogen atom has a duet of electrons, so the lone pairs reside on oxygen and form an octet on oxygen. For H—H—O bonding, the two covalent bonds again use 4 of the 8 e –, leaving 4 e– for 2 lone pairs. If these are placed on the oxygen atom as shown here, oxygen does not complete its octet and the central hydrogen atom has 4 e–, not a duet. This structure would violate the Lewis structure formalism. 8-27 8-29 8-31 The ions I– and Ca2+ have complete valence-shell octets. B3+ does not have an octet but rather the duet of the He atom. 8-33 (a) For a 1+ charge and 1 valence electron: (b) For a 3+ charge and no valence electrons: 8-35 (a) 3 valence e– (B) + 5 valence e– (N) = 8 valence e– (b) 1 valence e– (H) + 7 valence e– (F) = 8 valence e– (c) 6 valence e– (O) + 1 valence e– (H) + 1 e– (negative charge) = 8 valence e– (d) 4 valence e– (C) + 5 valence e– (N) + 1 e– (negative charge ) = 10 valence e– 8-37 (Step 1) The number of valence electrons in CO is Element C O Valence electrons per 4 + 6 = 10 atom (Step 2) There are only two atoms bonded together so neither is the central atom. C O 8-40 (Step 4) In this structure there are 8 electrons from three lone pairs and one bond pair. We need 6 more electrons (three pairs) to match the valence electrons determined in step 1. We add the lone pairs to the chlorine atom. Cl O (Step 5) This Lewis structure is complete. To indicate the charge on this ion we add brackets for the structure and the charge. Cl O (a) For CN– (Step 1) The number of valence electrons in CN– is Element Valence electrons per atom Gain of electron due to charge Total valence electrons C 4 + N 5 =9 +1 10 (Step 2) There are only two atoms bonded together so neither is the central atom. C N (Step 3) We complete the octet on the nitrogen atom by adding three lone pairs. C N (Step 4) In this structure there are 8 electrons from three lone pairs and one bond pair. We need 2 more electrons (one pair) to match the valence electrons determined in step 1. We add the lone pair to the carbon atom. C N (Step 5) To complete the octet on the carbon atom we convert two lone pairs on the N atom to give a triple bond between the nitrogen atom and the carbon atom. Finally, we add brackets to the structure and indicate the charge on this anion. The Lewis structure is now complete. C N C N 8-42 (a) CHF2Cl (Step 1) The number of valence electrons in CHF2Cl is Element C H 2F Cl Valence electrons 4 1 7 = 26 (2 7) per atom + + + (Step 2) Carbon has the most unpaired electrons (4) in its Lewis symbol and therefore has the highest bonding capacity and will be the central atom in the structure. The hydrogen, fluorine and chlorine atoms will each be bonded to the carbon. F H C Cl F (Step 3) We complete the octets on the chlorine and fluorine atoms by adding three lone pairs to each. F H C Cl F (Step 4) In this structure there are 26 electrons from nine lone pairs and four bond pairs. We do not need any more valence electrons in this structure. (Step 5) With carbon satisfied with its octet and the hydrogen satisfied with its duplet, the Lewis structure is complete. (b) CHBr3 (Step 1) The number of valence electrons in CHBr3 is Element C H 3Br Valence electrons per 4 1 + (3 7) = 26 atom + (Step 2) Carbon has the most unpaired electrons (4) in its Lewis symbol and therefore has the highest bonding capacity and will be the central atom in the structure. The hydrogen and bromine atoms will each be bonded to the carbon. Br H C Br Br (Step 3) We complete the octets on the bromine atoms by adding three lone pairs to each. Br H C Br Br (Step 4) In this structure there are 26 electrons from nine lone pairs and four bond pairs. We do not need any more valence electrons in this structure. (Step 5) With carbon satisfied with its octet and the hydrogen satisfied with its duplet, the Lewis structure is complete. (c) CH2Cl2 (Step 1) The number of valence electrons in CH2Cl2 is Element C 2H 2Cl Valence electrons per 4 (2 1) (2 7) = 20 atom + + (Step 2) Carbon has the most unpaired electrons (4) in its Lewis symbol and therefore has the highest bonding capacity and will be the central atom in the structure. The hydrogen and chlorine atoms will each be bonded to the carbon. H H C Cl Cl (Step 3) We complete the octets on the chlorine atoms by adding three lone pairs to each. H H C Cl Cl (Step 4) In this structure there are 20 electrons from six lone pairs and four bond pairs. We do not need any more valence electrons in this structure. (Step 5) With carbon satisfied with its octet and the hydrogen satisfied with its duplet, the Lewis structure is complete. 8-66 Because the double bond in NO is stronger than the 1.5 bond in NO2, the energy required to vibrate the N–O bond in NO is higher. 8-78 For HN3, hydroazoic acid (Step 1) The number of valence electrons is Element H 3N Valence electrons per 1 (3 5) = 16 atom + (Step 2) We are given that hydroazoic acid is a linear molecule with the connectivity of the atoms as H N N N (Step 3) We complete the octets on the rightmost nitrogen atom by adding three lone pairs. The duplet on the terminal H atom is already satisfied. H N N N (Step 4) In this structure there are 12 electrons from three lone pairs and three bond pairs. We need 4 more electrons (two pairs) to match the valence electrons determined in step 1. The middle nitrogen atoms do not have octets yet so we will add lone pairs. H N N N (Step 5) We can complete the octet for the nitrogen atoms by forming a triple bond between them. H N N N H N N N The electrons could also be distributed in two additional resonance forms that also complete the octet on all the atoms. The following resonance form is not valid because it has more than a duet for the H atom and has less than an octet for the N atom. H N N N H N N N H N N N 8-80 For urea, H2NC(O)NH2, we are given that there is a carbon–oxygen double bond. (Step 1) The number of valence electrons is Element C 4H O 2N Valence electrons per (1 4) (4 1) 6 + (2 5) = atom + + 24 (Step 2) We are given that urea has a carbon–oxygen double bond, and from the formula given, the connectivity of the atoms is O H N C N H H H (Step 3) We complete the octets on the nitrogen atoms by adding one lone pair to each, and we complete the octet on oxygen by adding three lone pairs. The duplet on the terminal H atom is already satisfied. O H N C N H H H (Step 4) In this structure there are 24 electrons from five lone pairs and seven bond pairs. This matched the number of electrons required for the structure. (Step 5) We can complete the octet for the carbon atom by forming a double bond between it and the oxygen atom. O H N O C N H H H N H H C N H H The electrons could also be distributed in two additional resonance forms that also complete the octet on all the atoms in which a double bond forms between nitrogen and carbon. O H N H C O N H H H N H C O N H H H N H C N H H 8-82 For nitrite ion, NO2– (Step 1) The number of valence electrons is Element Valence electrons per atom N 5 17 2O + (2 6) = Additional electrons for charge on + 1 anion Total valence electrons 18 (Step 2) Nitrogen has the most bonding capacity and is the least electronegative atom and so it is the central atom in the structure O N O (Step 3) We complete the octets on the oxygen atoms by adding three lone pairs to each. O N O (Step 4) In this structure there are 16 electrons from six lone pairs and two bond pairs. We need 2 more electrons (one pair) to match the number of electrons determined in step 1. These we add to the nitrogen atom. O N O (Step 5) We can complete the octet for the nitrogen atom by forming a double bond between the nitrogen and the oxygen atom. O N O O N O The electrons could also be distributed in one additional resonance form that also completes the octet on all the atoms by forming a double bond between nitrogen and the other oxygen atom. O N O O N O 8-85 For both HNC and HCN there are 10 valence electrons and the Lewis structures with formal charges are The formal charges are zero for all the atoms in HCN, whereas in HNC the carbon atom, with a lower electronegativity than N, has a –1 formal charge.







