Hazards of Industrial Gases
advertisement

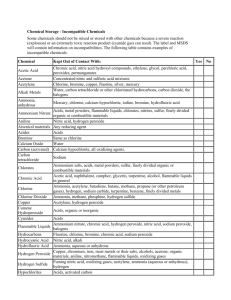
Hazards of Industrial Gases There are certain properties, hazards and precautions associated with the major industrial gases, as well as mixtures of same. These gases are: Acetylene, Air, Argon, Carbon Dioxide, Helium, Nitrogen, Nitrous Oxide, Oxygen, and Propane. Each of these gases has at least one of the following hazards: High Pressure Extreme cold Asphyxiating (Inert) Oxidizing Flammable NOTE: It should be stressed that more than one hazard can be associated with a particular gas. Hazard: High Pressure Most cylinder gases are under high pressure. Although most high pressure cylinders are filled to pressures between 2000 psi and 3000 psi, cylinders with pressure ratings up to 7500 psi are commonly available. Even lower pressure gases, such as Propane can be hazardous if the proper equipment is not utilized, or if leaks are encountered. Hazard: Extreme Cold Some users of Argon, Carbon Dioxide, Helium, Hydrogen, Nitrogen, or Oxygen receive these gases in cryogenic liquid form. Both the liquid and its vapor are extremely cold, ranging in temperature from -109F to -452F. The major hazards associated with these liquids are 1) danger of tissue destruction or severe frostbite 2) danger of asphyxiation and/or increased risk of fire hazard 3) build up of extremely high pressure from trapped evaporating liquids and 4) the embrittlement of many materials which may fracture at these temperatures. Hazard 3: Asphyxiating (Inert) Argon, Carbon Dioxide, Helium and Nitrogen are all inert gases. They will not support life and may cause asphyxiation by displacing the Oxygen in the air. To insure safety, the following precautions should always be observed: Areas where any inert gas is used or stored must be kept well ventilated. None of the inert gases can be adequately detected by any human sense; yet all can be inhaled as readily as air. If the Oxygen content drops below 19.5%, the reactions of personnel exposed will depend upon the response of the individual involved. However, one should not expect to experience a choking sensation, rather, there may be an almost immediate loss of consciousness. Before entering confined spaces which can be Oxygen deficient atmospheres, you should first check the Oxygen levels with an Oxygen analyzer. If there is the possibility of excessive Carbon Dioxide in a confined space, it is not sufficient to merely check the Oxygen concentration. Carbon Dioxide concentrations already in the blood stream, regulate certain bodily functions and excess concentrations may act as a depressant on the central nervous system. Hazard 4: Oxidizing Two industrial gases that are recognized as oxidizers are Oxygen and Nitrous Oxide. Materials which normally do not burn in air may burn with explosive violence in an Oxygen enriched atmosphere. To insure safety, the following precautions must always be followed: Always refer to Oxygen by its name. NEVER call it "air" and NEVER use it as a substitute for compressed air. Keep all organic materials, especially oil, grease, wood, cloth, or asphalt, away from contact with Oxygen. Never attempt to lubricate any equipment used in Oxygen service. Arrange for repairs through your supplier. When liquid Oxygen is spilled or vented, a white cloud of condensed moisture results. Standing in or near this cloud will saturate clothing with Oxygen, making it extremely subject to rapid and intense burning. Should this happen, personnel involved should move to a clear area and avoid smoking, open flames, or other sources of ignition for at least one half hour to allow clothing to adequately air out. Prevent spillage onto asphalt or oil contaminated concrete, soil or other surfaces. Hazard 5: Flammable Acetylene, Hydrogen, and Propane are flammable gases. If any of these gases mix with air or Oxygen, the mixture is subject to ignition or explosion if exposed to an ignition source. The concentrations needed are low. 2.2% in air for Propane, 2.5% for Acetylene, and 4% for Hydrogen. In order to avoid accidents, observe the following safety precautions: Store cylinders containing flammable gases outdoors or in well ventilated areas. Away from oxidizers and never near sources of heat, flames, or sparks. Never use a flame to detect flammable gas leaks. Never permit the delivery pressure of Acetylene to exceed 15 psig. Never attempt to transfer Acetylene into another cylinder or mix any gas with Acetylene in a cylinder. Acetylene should not be exposed to copper, silver, mercury, their salts, compounds and alloys. Explosive acetylide compounds may be formed. Hydrogen gas burns with an almost invisible pale blue flame. If there is suspicion that Hydrogen is burning, approach with a broom extended into the suspected flame region to confirm the presence of burning. If the Hydrogen is burning, the broom will ignite. Call the fire department as soon as a fire is detected, unless the fire can be easily quenched by shutting off the source of the gas and the fire has not spread to adjacent areas or articles. Propane is much heavier than air, therefore, it will flow to low points, to be ignited at distances that may be quite far