Atomic Structure Worksheet: Protons, Neutrons, Electrons
advertisement
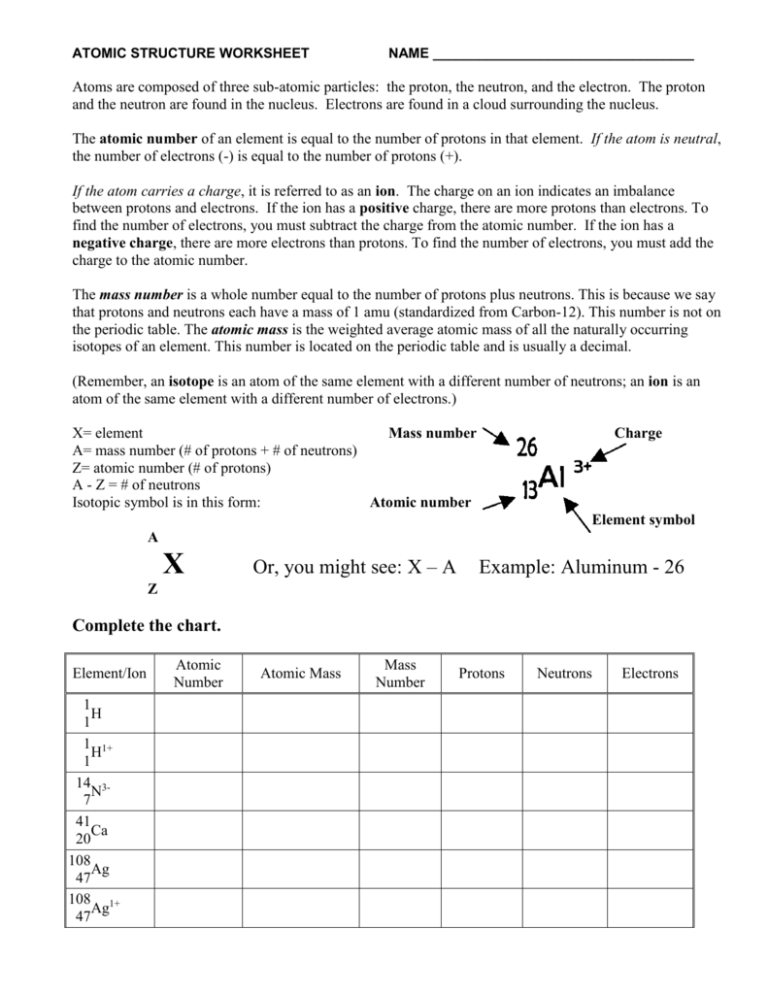
ATOMIC STRUCTURE WORKSHEET NAME __________________________________ Atoms are composed of three sub-atomic particles: the proton, the neutron, and the electron. The proton and the neutron are found in the nucleus. Electrons are found in a cloud surrounding the nucleus. The atomic number of an element is equal to the number of protons in that element. If the atom is neutral, the number of electrons (-) is equal to the number of protons (+). If the atom carries a charge, it is referred to as an ion. The charge on an ion indicates an imbalance between protons and electrons. If the ion has a positive charge, there are more protons than electrons. To find the number of electrons, you must subtract the charge from the atomic number. If the ion has a negative charge, there are more electrons than protons. To find the number of electrons, you must add the charge to the atomic number. The mass number is a whole number equal to the number of protons plus neutrons. This is because we say that protons and neutrons each have a mass of 1 amu (standardized from Carbon-12). This number is not on the periodic table. The atomic mass is the weighted average atomic mass of all the naturally occurring isotopes of an element. This number is located on the periodic table and is usually a decimal. (Remember, an isotope is an atom of the same element with a different number of neutrons; an ion is an atom of the same element with a different number of electrons.) X= element Mass number A= mass number (# of protons + # of neutrons) Z= atomic number (# of protons) A - Z = # of neutrons Isotopic symbol is in this form: Atomic number Charge Element symbol A X Or, you might see: X – A Example: Aluminum - 26 Z Complete the chart. Element/Ion 1 H 1 1 1+ H 1 14 3N 7 41 Ca 20 108 Ag 47 108 1+ Ag 47 Atomic Number Atomic Mass Mass Number Protons Neutrons Electrons Define the following: Atomic Number _______________________________________________________________________ Mass Number _________________________________________________________________________ Atomic Mass _________________________________________________________________________ Isotope _______________________________________________________________________________ Ion ___________________________________________________________________________________ Isotope Name Isotope Symbol Protons Number of… Neutrons Electrons Atomic Number Mass Number 16 34 20 40 Argon-40 Argon-38 Bismuth-209 57 26 Fe 209 82 Pb 28 37 30 34 28 48 37 Aluminum-27 Manganese-55 11 12 SUMMARIZE: How do you find the number of protons for any atom? How do you find the number of neutrons for any atom? How do you find the number of electrons for any atom? 11










