Name

.
Name_________________________________________________
UNIT 10: Solutions Practice Problems
1. Two liquids that can be mixed together but separate shortly after are:
Period_______
2. A mixture containing particles that settle out if left undisturbed is a:
3. A heterogeneous mixture of intermediate sized particles is a:
4. More solute can be dissolved in a _________________________________ solution:
5. A substance that dissolves in a solvent is said to be:
6. A _____________________________ contains the maximum amount of dissolved solute for
a given amount of solvent.
7. Two liquids that are soluble in each other in any proportion are said to be:
8. An example of a suspension is: a. blood b. gelatin
9. Illustrated is a phenomenon know as the: c. milk d. muddy water
10. A substance that does not dissolve in a solvent is said to be:
11. The image illustrates what is formed from a ______________________________________ solution.
12. What law states that at a given temperature the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid?
13. The erratic movement of colloid particles is called:
14. A ______________________________ solution contains more dissolved solute than a saturated solution
at the same temperature.
15. The liquid illustrated is an example of a/an:
16. __________________________________________________ solutions are very unstable.
17. When a liquid is insoluble in another liquid, the liquids are said to be _________________________.
18. A substance that does not dissolve in a solvent is said to be _________________________ in that solvent.
19. The maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure is called __________________________________.
20. A measure of the amount of solute dissolved in a specific amount of solvent or solution is called the
____________________________________ of the solution.
21. In kidney dialysis, the diffusion of solvent particles takes place across a semipermeable membrane from an area of ___________________________ to ____________________ solvent concentration.
22. When solute particles are added to a pure solvent in a closed container at a constant temperature and pressure, the vapor pressure __________________________________.
23. The type of solution formed by creek water after heavy rain is called _____________________________.
24. The process of surrounding solute particles with solvent particles to form a solution is called
____________________________________________.
25. Air contains oxygen as the solute and ________________________________________ as the solvent.
26. The process illustrated is called ___________________________________.
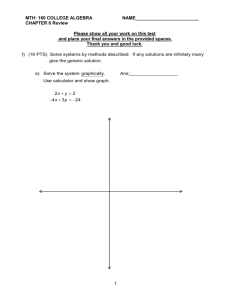
27. Calculate the mass of nitric acid dissolved in 0.48 kg of water.
28. Calculate the molarity of the solution.
29. When a liquid is insoluble in another liquid, the liquids are said to be ____________________________.
30.
Is it possible to have a colloidal dispersion from one gas to another? Explain why or why not.
31.
Why does a soft drink lose its fizz after it has been opened and left with a lid on the container?
32.
If 0.85 g of gas at 4.0 atm of pressure dissolves in 1.0 L of water at 25°C, how much will dissolve in 1.0 L of water at 1.0 atm of pressure and the same temperature?
33. Calculate the moles of camphor dissolved in phenol. The molar mass of camphor is 152 g/mol.
34.
The solubility of a gas is 0.55 g/L at 8.0 atm pressure. What will be the solubility of the gas at 5.0 atm partial pressure?
35.
If 0.80g of sulfur dioxide at 10.00 atm pressure ( P
) dissolves in 5.00L of water at 25.0°C, how much of it will dissolve in 1 L of water at 9.00 atm pressure ( P ) and the same temperature?
36. In the lab, a chemist adds 3.6 grams of sodium chloride to 100.0 g of water. What is the molarity of the solution?
37. The maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure is called __________________________________.
38. What volume, in milliliters, of calcium chloride stock solution would you use to make 0.50 L of 0.300 M of calcium chloride solution?
61.
39. A substance that does not dissolve in a solvent is said to be _______________________ in that solvent.
40. The type of solution formed in a creek after a heavy rain is called a ____________________________.
41.
What is the difference between suspended and colloid particles?
42. A measure of the amount of solute dissolved in a specific amount of solvent or solution is called the
_______________________________ of the solution.
43. The temperature difference between a solution’s boiling point and a pure solvent’s boiling point is called the
_________________________________.
44. The temperature difference between a solution’s freezing point and a pure solvent’s freezing point is called the ___________________________________.
45. What is the relationship between temperature and solubility of substances?
46.
Explain why a soft drink loses its fizz after it has been open and left without a lid on its container.
47.
Explain why boiling point elevation is considered a colligative property.
48.
Explain why sand particles are insoluble in water.
49.
Describe the hydration process.
50.
Why is it not possible to have a colloidal dispersion from one gas to another?
51. Explain the random movement of pollen grains in water
Unit 10: Solutions Practice Problems
Answer Section
TRUE/FALSE
1. ANS: T
NAT: UCP.3
PTS: 1 DIF: Bloom's Level 1
2. ANS: F
Carbonated water is a solution of carbon dioxide in water.
PTS: 1
3. ANS: T
NAT: UCP.2
DIF: Bloom's Level 1
PTS: 1
NAT: UCP.2
DIF: Bloom's Level 1
DIF: Bloom's Level 2 4. ANS: T
NAT: UCP.2
5. ANS: T
NAT: UCP.2
PTS: 1
PTS: 1
6. ANS: T
NAT: UCP.1 | UCP.2
PTS: 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
7. ANS: F
A mixture is a combination of TWO or more pure substances in which each pure substance retains its individual chemical properties.
PTS: 1 DIF: Bloom's Level 1 NAT: UCP.2 | B.2
8. ANS: F
A colloid is a heterogeneous mixture of intermediate sized particles.
PTS: 1 DIF: Bloom's Level 1 NAT: UCP.2
9. ANS: T
NAT: UCP.1 | B.2
PTS: 1 DIF: Bloom's Level 3
10. ANS: F
A CONCENTRATED solution contains large amounts of solute.
PTS: 1
11. ANS: T
NAT: UCP.2
DIF: Bloom's Level 2
PTS: 1
NAT: UCP.1 | UCP.2
DIF: Bloom's Level 2
DIF: Bloom's Level 1 12. ANS: T
NAT: UCP.1 | UCP.2
PTS: 1
PTS: 1 13. ANS: T
NAT: UCP.2
14. ANS: T
NAT: UCP.2
PTS: 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
15. ANS: F
In a saturated solution, the amount of dissolved solute particles is maximum for a given amount of solvent at constant temperature and pressure.
PTS: 1 DIF: Bloom's Level 1 NAT: UCP.2 | B.3
16. ANS: F
Carbonated water is a solution of carbon dioxide in water.
PTS: 1 DIF: 1 REF: Page 454
OBJ: 15.1.1 Describe the characteristics of solutions and identify the various types.
NAT: UCP.1 | B.2 TOP: Describe the characteristics of solutions and identify the various types.
KEY: Solutions MSC: 1
NOT: When carbon dioxide gas is mixed with water, it forms a carbonate of water, which is called carbonated water.
17. ANS: F
Gypsum is insoluble in water. Solvation takes place only when the force of attraction between solvent particles and the solute particles is greater than the attractive forces between the solute particles.
PTS: 1 DIF: 1 REF: Page 455
OBJ: 15.1.2 Relate intermolecular forces and the process of solvation.
NAT: B.1 | B.4
KEY: Solvation
TOP: Relate intermolecular forces and the process of solvation.
MSC: 2
NOT: The attractive forces among the solute ions are stronger than the attractive forces exerted by the water molecules.
18. ANS: T
One sugar molecule has eight O–H bonds, which provide the site for hydrogen bonding with water.
PTS: 1 DIF: 1 REF: Page 456
OBJ: 15.1.2 Relate intermolecular forces and the process of solvation.
NAT: B.1 | B.4
KEY: Solvation
TOP: Relate intermolecular forces and the process of solvation.
MSC: 2
NOT: Hydrogen bonds have forces of attraction due to which water molecules collide with the outer surface of the solute particles and lead to solvation.
19. ANS: F
In a saturated solution, the amount of dissolved solute particles is maximum for a given amount of solvent at a constant temperature and pressure.
PTS: 1 DIF: 1 REF: Page 458
OBJ: 15.1.3 Define solubility and identify factors affecting it. NAT: B.2
TOP: Define solubility and identify factors affecting it.
MSC: 1
KEY: Saturated solution
NOT: In a saturated solution, solute particles can be added into the solvent particles by raising the temperature.
20. ANS: F
Solvation in water is called hydration.
PTS: 1 DIF: 1 REF: Page 455 | Page 457
OBJ: 15.1.2 Relate intermolecular forces and the process of solvation.
NAT: B.1 | B.4
KEY: Solvation
TOP: Relate intermolecular forces and the process of solvation.
MSC: 1
NOT: Solubility refers to the maximum amount of solute that will dissolve in a given amount of solvent at a specified temperature and pressure.
MULTIPLE CHOICE
1. ANS: A PTS: 1
NAT: UCP.2 | UCP.3 | B.3
2. ANS: D
NAT: UCP.2 | B.3
PTS: 1
3. ANS: A
NAT: UCP.2 | B.3
4. ANS: D
NAT: UCP.2 | B.3
5. ANS: D
NAT: UCP.2
PTS: 1
PTS: 1
PTS: 1
6. ANS: A
NAT: UCP.2 | UCP.3
PTS: 1
7. ANS: C
NAT: UCP.2 | UCP.3
PTS: 1
8. ANS: D
NAT: UCP.2
PTS: 1
9. ANS: D
NAT: UCP.1 | UCP.2
PTS: 1
PTS: 1 10. ANS: A
NAT: UCP.2
11. ANS: B
NAT: UCP.2 | B.3
PTS: 1
PTS: 1 12. ANS: C
NAT: B.3
13. ANS: A
NAT: B.3 | B.4
14. ANS: B
NAT: UCP.2 | B.3
PTS: 1
PTS: 1
15. ANS: A
NAT: UCP.2
16. ANS: B
NAT: UCP.2
PTS: 1
PTS: 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
DIF: Bloom's Level 1
DIF: Bloom's Level 3
DIF: Bloom's Level 3
DIF: Bloom's Level 1
DIF: Bloom's Level 2
DIF: Bloom's Level 1
DIF: Bloom's Level 1
DIF: Bloom's Level 3
DIF: Bloom's Level 2
DIF: Bloom's Level 2
COMPLETION
17. ANS: immiscible
PTS: 1 DIF: 1 REF: Page 454
OBJ: 15.1.1 Describe the characteristics of solutions and identify the various types.
NAT: UCP.1 | B.2 TOP: Describe the characteristics of solutions and identify the various types.
KEY: Immiscible MSC: 1
18. ANS: insoluble
PTS: 1 DIF: 1 REF: Page 454
OBJ: 15.1.1 Describe the characteristics of solutions and identify the various types.
NAT: UCP.1 | B.2 TOP: Describe the characteristics of solutions and identify the various
types.
KEY: Insoluble MSC: 1
19. ANS: solubility
PTS: 1 DIF: 1 REF: Page 457
OBJ: 15.1.3 Define solubility and identify factors affecting it. NAT: B.2
TOP: Define solubility and identify factors affecting it.
MSC: 1
KEY: Solubility
20. ANS: concentration
PTS: 1 DIF: 1 REF: Page 462
OBJ: 15.2.1 State the concentrations of solutions in different ways.
NAT: UCP.3 | B.2 TOP: State the concentrations of solutions in different ways.
KEY: Concentration MSC: 1
21. ANS: higher, lower
PTS: 1 DIF: 1 REF: Page 475
OBJ: 15.3.2 Describe four colligative properties of solutions. NAT: UCP.2 | B.2
TOP: Describe four colligative properties of solutions.
MSC: 2
KEY: Osmosis
22. ANS: decreases lowers
PTS: 1 DIF: 1 REF: Page 472
OBJ: 15.3.2 Describe four colligative properties of solutions. NAT: UCP.2 | B.2
TOP: Describe four colligative properties of solutions.
MSC: 2
KEY: Vapor pressure
23. ANS: suspension
PTS: 1
NAT: B.2
DIF: 1 REF: Page 476
OBJ: 15.4.1 Identify the properties of suspensions and colloids.
TOP: Identify the properties of suspensions and colloids.
KEY: Suspensions MSC: 1
24. ANS: solvation
PTS: 1 DIF: 1 REF: Page 455
OBJ: 15.1.2 Relate intermolecular forces and the process of solvation.
NAT: B.1 | B.4 TOP: Relate intermolecular forces and the process of solvation.
KEY: Solvation MSC: 1
25. ANS: nitrogen
PTS: 1 DIF: 1 REF: Page 454
OBJ: 15.1.1 Describe the characteristics of solutions and identify the various types.
NAT: UCP.1 | B.2 TOP: Describe the characteristics of solutions and identify the various types.
KEY: Solutions MSC: 1
SHORT ANSWER
26. ANS:
Solvation
PTS: 1
27. ANS:
36 g
PTS: 1
28. ANS:
0.2 / 0.5 = 0.4 M
PTS: 1
DIF: Bloom's Level 3
DIF: Bloom's Level 3
NAT: UCP.2
NAT: UCP.3
DIF: Bloom's Level 3 NAT: UCP.3 | UCP.5 | A.1
29. ANS:
Immiscible
PTS: 1
PTS: 1
B.6
DIF: Bloom's Level 3 NAT: UCP.2
30. ANS:
No. Two gases would result in a homogenous mixture. A colloidal mixture is heterogeneous.
DIF: Bloom's Level 3 NAT: UCP.2 | UCP.5 | B.3 |
31. ANS:
The fizz is dissolved carbon dioxide which has been dissolved into the drink at a pressure much higher than atmospheric pressure. Opening the container and leaving it exposed to air at normal pressure causes the dissolved carbon dioxide to escape into the air. The loss of carbon dioxide causes the drink to lose its fizz.
PTS: 1 DIF: Bloom's Level 3 NAT: UCP.2 | B.3 | E.2
32. ANS:
0.21 g/L
0.85 g / 1.0 L * 1.0 atm / 4.0 atm = 0.21 g/L
PTS: 1 DIF: Bloom's Level 3
33. ANS:
0.0522 mol
PTS: 1 DIF: Bloom's Level 3
34. ANS:
0.34 g/L
PTS: 1 DIF: Bloom's Level 3
35. ANS:
S2 = .14g/L
PTS: 1 DIF: Bloom's Level 3
36. ANS:
.077 mol NACL / .1000 kg H
2
0 = .77 mol/kg
NAT: UCP.3 | A.1
NAT: UCP.3 | A.1
NAT: UCP.3 | A.1
NAT: UCP.3 | A.1
PTS: 1
37. ANS:
Solubility
PTS: 1
DIF: Bloom's Level 3
DIF: Bloom's Level 3
38. ANS:
75 ml
V1 = (0.50 L) x 0.300 M / 2.00 M = .075 L = 75 ml
PTS: 1 DIF: Bloom's Level 3
NAT: UCP.3 | A.1 | B.3
NAT: UCP.2 | UCP.3 | B.3
NAT: UCP.3 | B.3
39. ANS:
Insoluble
PTS: 1
40. ANS:
Suspension
PTS: 1
DIF: Bloom's Level 3 NAT: UCP.2 | B.3
DIF: Bloom's Level 3 NAT: UCP.2 | D.3
41. ANS:
Suspended particles are much larger than colloid. Suspension particles can be separated by settling or filtration where colloid particles cannot.
PTS: 1 DIF: Bloom's Level 3 NAT: UCP.2
42. ANS:
Concentration
PTS: 1 DIF: Bloom's Level 3
43. ANS:
Boiling Point Elevation
PTS: 1 DIF: Bloom's Level 1
44. ANS:
Freezing Point Depression
PTS: 1 DIF: Bloom's Level 1
NAT: UCP.3 | B.3
NAT: UCP.2 | B.2 | B.3
NAT: UCP.2 | B.2 | B.3
45. ANS:
The relationship between temperature and solubility varies from one solute to another. The solubility increases in most substances with an increase in temperature, but in a few substances, the solubility decreases with an increase in temperature.
PTS: 1 DIF: 1 REF: Page 458
OBJ: 15.1.3 Define solubility and identify factors affecting it. NAT: B.2
TOP: Define solubility and identify factors affecting it.
Temperature
KEY: Solubility |
MSC: 1
46. ANS:
The fizz of the beverage is carbon dioxide dissolved into the soft drink at a pressure much higher than atmospheric pressure. Opening the container and leaving it exposed to air at normal atmospheric pressure causes the dissolved carbon dioxide to bubble up in the soft drink allowing it to escape into the air. The loss of dissolved carbon dioxide causes the drink to lose its carbonation or fizz.
PTS: 1 DIF: 1 REF: Page 460
OBJ: 15.1.3 Define solubility and identify factors affecting it. NAT: B.2
TOP: Define solubility and identify factors affecting it.
MSC: 2
KEY: Pressure | Solubility
47. ANS:
The boiling point of a solution is usually higher than the boiling point of the solvent. This is because the solute lowers the vapor pressure of the solution below the atmospheric pressure. This prevents the solution from boiling at the boiling point of the solvent. Additional solute will create a greater disparity between the vapor pressure of the solution and the atmospheric pressure resulting in an elevated boiling point. Since the elevation of the solution’s boiling point is solely a result of the amount of solute particles, boiling point elevation is considered a colligative property.
PTS: 1 DIF: 2 REF: Page 471 | Page 472
OBJ: 15.3.1 Explain the nature of colligative properties. NAT: UCP.2 | B.2
TOP: Explain the nature of colligative properties.
MSC: 2
KEY: Colligative property
48. ANS:
Sand particles are insoluble in water because the attractive forces between sand particles are greater than the attractive force between water and sand particles. The attractive forces of sand particles are so strong that they cannot be overcome by the attractive forces exerted by water molecules. Therefore, sand particles are insoluble in water.
PTS: 1 DIF: 2 REF: Page 455
OBJ: 15.1.2 Relate intermolecular forces and the process of solvation.
NAT: B.1 | B.4
KEY: Solvation
TOP: Relate intermolecular forces and the process of solvation.
MSC: 2
49. ANS:
When a crystal of salt is placed in a beaker of water, molecules of water collide with the solute particles due to the polarity of water. Water molecules have partially positive and negative charged ends. These charged ends attract the oppositely charged solute particles because the attraction between water molecule and solute ions is greater than the attraction among the solute ions in the crystal. Hydration is the result of collision between water and the solute particles.
PTS: 1 DIF: 2 REF: Page 455
OBJ: 15.1.2 Relate intermolecular forces and the process of solvation.
NAT: B.1 | B.4 TOP: Relate intermolecular forces and the process of solvation.
KEY: Hydration MSC: 2
50. ANS:
It not possible to have a colloidal dispersion from one gas to another because the two gases would result in the formation of a homogenous molecular mixture. A colloidal solution is a heterogeneous mixture.
PTS: 1 DIF: 2 REF: Page 476
OBJ: 15.4.1 Identify the properties of suspensions and colloids.
NAT: B.2 TOP: Identify the properties of suspensions and colloids.
KEY: Colloids MSC: 2
51. ANS:
Pollen grains have polar or charged atomic groups on their surfaces. These areas on the surface of pollen grains attract the positively or negatively charged areas of water resulting in the formation of electrostatic layers around the particles. The layers repel each other when the pollen particles collide and, thus, the particles remain in the colloid. This erratic movement of pollen grains in water is called Brownian motion.
PTS: 1 DIF: 2 REF: Page 478
OBJ: 15.4.3 Explain the electrostatic forces in colloids. NAT: UCP.2 | B.1 | B.2 | B.4
TOP: Explain the electrostatic forces in colloids.
MSC: 2
KEY: Brownian motion