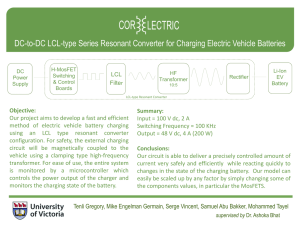
Batteries
advertisement

Batteries Categories Primary Battery Can produce current immediately on assembly Disposable Used in low current drain or intermittently Can NOT be recharged – the chemical reactions are not reversible and active materials may not return to their original forms Lose 8-20% of charge per year at 20-30oC Store at lower temperatures, but do NOT freeze Types Zinc-Carbon Zinc-Chloride (Heavy Duty) Alkaline – Zinc-Manganese Dioxide Warning: container polarity reversed between old and new varieties Others – Nickel Oxyhydroxide, Li-CuO, LiFeS2, Mercury-oxide, Siver-oxide Secondary Battery Must be charged before use – assembled with active materials in a discharged state Rechargeable Cycle Types Starting Battery Thin plates designed to maximize surface area/current output Don’t discharge more that 20% Deep Cycle Battery Thick, heavy plates designed for repeated heavy discharges Designed for up to 80% discharge Cell Types Wet Cell or Flooded Cell Lead-Acid Battery Plates surrounded by sulfuric acid electrolyte Positive - chocolate brown (Lead Oxide alloy) Negative – slate gray (Lead alloy) Separator Sulphation Produce Hydrogen Gas when charging Heavy Spills Sealed vs Vented Valve Regulated Lead-Acid Battery (VRLA) Flat plate or spiral Electrolyte is immobilized Gel Cell – semi-solid electrolyte Absorbed Glass Mat (AGM) - electrolyte in a special fiberglass matting Heavy Greater resistance to temperature, shock or vibration No need to keep upright Slower Discharge Longer life Dry Cell Nickel-Cadmium (NiCd) Lose 10% of charge in first 24 hours then 10% per month Rated for 1000 cycles Memory effect – crystals building up Discharge completely before recharging Environmental Hazard due to Cadmium Nickel –Zink (NiZn) – not commercially viable yet Nickel Metal Hydride (NiMH) Regular vs Slow Discharge Eneloop discharge at 15% per year Rated at 500-1000 cycles Lithium-Ion (Li-ion) Expensive High energy density – used in laptops, digital cameras, camcorders, cell phones Explodes if short circuited Molten Salts Uses molten salts as an electrolyte – hard to keep up to temperature Reserve Activated by adding electrolyte Impact breaking a capsule Sea water activated device Short service life after long-term storage Capacity – The amount of electrical Charge it can store Standard Capacity – The constant current a battery can supply for 20 hours at 68oF (20oC) X 20 Example: A battery rated at 100 Ah will deliver 5 amps over a 20-hour period. Higher discharge rates deliver less energy Cold Cranking Amps – How much amperage can be supplied by the battery at 0oF for 30 seconds before the battery voltage drops to 7.2 volts Charging Measuring Depth of Discharge (DoD) Specific Gravity (corrected for temperature) Load Testers Generally, for 6-cell Lead-Acid Batteries Open-circuit at full charge: 12.6v (2.1v per cell) Continuous-Preservation (Float) Charging: 13.4v gelled electrolyte 13.5v AGM 13.9v flooded cell Precise float voltage is critical to longevity Insufficient voltage causes sulfation Excessive voltage causes corrosion and electrolyte loss Fast vs Slow Charge Quick charge only scratches the surface and can decrease the life of the battery Slow charge penetrates the plates further and is better for the battery Smart Chargers Desulfonators For wet-cell lead-acid batteries Pulse conditioning that breaks down the sulfate crystals on the plates Off-gassing When charging, a flooded battery gives of hydrogen gas – explosive Additives Epsom Salts – reduced the internal resistance in weak or damaged battery and may slightly extend life EDTA – dissolves sulfate deposits in heavily discharged plates but the dissolved material is no longer available to participate in the charge discharge cycle. It also forms organic acids which accelerates corrosion of the plates and connectors Connections Terminal lugs Copper Spade Andersen Connectors Maintenance Terminal Corrosion – Corrosion of the positive terminal is caused by electrolysis, due to mismatch of metal alloys used in manufacturing Cleaning Sealing – Petroleum jelly or commercial sprays Clean up – Baking soda Charge Lead-Acid batteries every 6 months or when shows 70% capacity (charge) Charge NiCd and NiMH batteries at least once a year. Condition the battery (charge and discharge) before heavy use Avoid freezing but store in cool, dry place