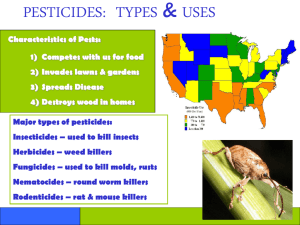
PESTICIDES: An Overview
advertisement

IB 486: Pesticide Toxicology I. Introduction and History PESTICIDES: An Overview A pesticide is any chemical marketed for the purpose of killing or repelling a pest. Pests include insects, mites, plants, algae, fungi, birds, and mammals (usually rodents; occasionally larger mammals, such as coyotes). The pesticides that kill these organisms are known, respectively, as insecticides, acaricides (or miticides), herbicides, algicides, fungicides, avicides, and rodenticides. The term 'pesticide' is extremely general, since it includes all of the separate categories. Moreover, for regulatory purposes, chemicals that are intended to repel organisms without killing them are also considered pesticides and are regulated in the same way. Chemicals that kill microbes are generally not included under pesticides, but are given the separate category of antibiotics. There is some overlap, especially among fungicides, which are often considered antibiotics if taken by humans or domestic animals, but as pesticides if applied to crops. Nomenclature There are several different ways of identifying a specific pesticide. First, there is a chemical formula, which can usually be drawn in a 2-dimensional form that identifies all of the atoms in the chemical, as well as the bonds between them. In some cases a 3-dimensional structure is required to identify enantiomers uniquely, but chemists have devised 2-dimensional notations to provide this 3-dimensional information. Because structures do not change, and because most pesticides are not so complex that our understanding of their structure is likely to change over time, the 2-dimensional structural representation of a pesticide is usually the best single method of identification. Second, there is a verbal equivalent of this structure, constructed according to strict rules. Although theoretically unambiguous, these rules – and therefore the chemical names - change over time. For example, p,p'-DDT was initially assigned the chemical name of 4,4'-dichlorodiphenyltrichloroethane, which led to its common, or generic, name of DDT. Figure 1: 1 Subsequently it became known as 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane. However, the same structure can be designated1 as: 1,1'-(2,2,2-trichloroethylidene) bis (4-chlorobenzene); 1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane; 1,1,1-trichloro-2,2-di (4-chlorophenyl) ethane; 1,1,1-trichloro-2,2-di(p-chlorophenyl) ethane; 1,1-bis(4-chlorophenyl)-2,2,2-trichlorethane; 1,1-bis(p-chlorophenyl)-2,2,2-trichlorethane; 2,2,2-trichloro-1,1-bis (4-chlorophenyl) ethane; 2,2-bis(p-chlorophenyl)-1,1,1-trichloroethane. which surely demonstrates that a picture is worth quite a few words! Third, each pesticide has a common, or generic, name. The generic names may be suggested by the manufacturer, but are officially assigned by the International Union of Pure and Applied Chemists, IUPAC, and by the International Organization for Standardization (ISO)2. Chemical Abstracts (CAS) also designates common names. These generic names may also differ between countries. Often the change is trivial, designed only to keep a similar pronunciation, but occasionally (especially for older pesticides) names in different countries may bear no resemblance to each other. The generic name may be thought of as a 'nickname'. It is quite durable, as shown by the fact that DDT is still called by its original acronym, even though the chemical name changed over 20 years ago! Finally, each commercial pesticide quickly acquires a brand name, which belongs to the manufacturer of a particular formulation. In fact, the brand name often precedes the generic name, because the company that patents the synthetic process usually assigns a brand name to the chemical well before it is actually marketed. The same brand name may, over time, be used for different pesticides, or different combinations of pesticides, and a single manufacturer may use several brand names for the same active ingredient, depending on the exact pesticide formulation. In addition, different manufacturers are likely to use very different names for the same pesticide, even in very similar formulations. Figure 2: Parathion-ethyl 1 according to: http://www.nsc.org/library/chemical/ddt.htm 2 A compendium of pesticide names according to IUPAC, ISO and CAS can be found at http://www.alanwood.net/pesticides/ 2 For example, the organophosphate insecticide ethyl parathion has been known by all of the following brand names: Alkron, Alleron, Corothion, Folidol, Folidol E-65, Genthion, Geofos, Lethalaire, Niran, Orthophos, Paradusto, Paraspra, Penncap-E, Thiophos (USSR), Vapophos, Aphemite, Bladan, Bladan F, Corthione, Danthion, DNTP, DPP, E 605, Ecatox, Ekatox, Etilon, Fosferno, Fosfex, Fosfive, Fosova, Fostern, Genthion, Kolphos, Kypthion, Lirothion, Murfos, Niran, Nitrostigmine, Nourithion, Oleofos 20, Oleoparathion, parathene, Parawet, Pestox plus, Phoskil, Phosphemol, Phosphenol, Phosphostigmine, Rhodiatox, SNP, Soprathion, Stathion, Strathion, Sulphos, Tiofos, Thiophos, Tox 47, Vapophos ... Names for parathion in mixtures include: Bladen extra (with methyl parathion), Malatox (with malathion); Sopragram (with lindane); Tamaron (with acephate-methyl). Given this plethora of names, how can one be sure of identifying one's favorite pesticide in all its guises? The answer is -- with difficulty. In fact, over time, the only unique designation of a chemical (other than its structure) seems to be its Chemical Abstracts (CAS) Number. The CAS number is designated by Chemical Abstracts, and is changed only in the rare case where one chemical unexpectedly turns out to be identical with another. So, for the two examples I have used: DDT is both dichlorodiphenyltrichloroethane and 1,1,1-trichloro2,2-bis(p-chlorophenyl)ethane, and has any number of brand names. But there is only one CAS # associated with all of the names: 50-29-3. Similarly, parathion-ethyl may be called parathion, ethyl parathion, or any one of dozens of brand names: but the single CAS number associated with them all is 56-38-23. 2. HISTORY: DDT as icon The 10 years following World War II saw the introduction of numerous synthetic chemicals, in everincreasing quantity. Monsanto’s catchy slogan of “better living through chemistry” seemed a truthful description of the modern era. Moreover, the new insecticides were so much less deadly than the older compounds that they seemed harmless, and so effective against so many species of insects that entomologists seriously discussed their own obsolescence. Even though signs of environmental damage due to organochlorine insecticides were recognized as early as the mid-1950s, they were considered isolated examples without general application. Moreover, such was the enthusiasm for the new chemicals that it was often difficult to publish data that pointed out harmful effects. And DDT, as the first of the wonder pesticides, was the icon for all of them. For over 60 years, it has been the single most-discussed pesticide: most praised, most vilified, most regretted – and certainly best known. Even today it remains a target for wishful thinkers: e.g., in January of 2005, Nicholas Kristoff, an Op-Ed columnist for the NY Times, wrote a column condemning the banning of DDT (which occurred in 1972). He argued that banning DDT was the selfish luxury of a rich nation, and condemned millions in poor nations to the scourges of insect-borne disease. This attitude completely ignores not only the inescapable reality of insecticide resistance to DDT, but also the fact that DDT has been, and continues to be, used by developing nations. Unfortunately it cannot exterminate malaria-carrying mosquitos, which long ago developed considerable resistance to DDT. The World Health Organization (WHO) did not shift from DDT to dieldrin and other insecticides because of environmental concerns - the shift preceded awareness of any environmental damage – but because the incidence of malaria was 3 It is quite feasible to use the CAS number to search for a pesticide using Google. 3 increasing as mosquitos became resistant to DDT. And nations that continued to use DDT have had to apply larger and larger quantities, with less and less effectiveness. DDT is still used today, especially (but not only) for public health purposes. Only in the 21st century has a world-wide treaty to phase out all POPs (persistent organic pollutants), including DDT, been ratified by enough countries to take effect. To understand the level of emotion attaching to DDT, one must consider the pre-WW II world of pest control. The noted pesticide toxicologist RL Metcalf, who studied insecticides from the early 1940s until his death in 1998, wrote: DDT’s insecticidal properties were discovered in a world just plunged into the catastrophe of World War II. Military sanitarians had grim memories of the ravages of louse-borne typhus which, in World War I, had been a critical factor in the fortunes of the Central Powers in the Balkans and on the Eastern Front. The rapid enlargement of the conflict of World War II into Greece, North Africa, and later to the south Pacific focused attention on mosquito-borne diseases such as malaria, dengue, and filariasis. Thus there was immediate interest in a cheap synthetic chemical which it was safe to apply directly to human bodies and was so persistent that it killed mosquitos for months after application. DDT’s use resulted in the conquest of typhus in Naples in 1943 and in the eradication of malaria from the Latina Province of Italy and in Sardinia beginning in 1945. For the first time in history, man had a safe, cheap, and durable weapon which could not only control but could often eradicate enclaves of vector-borne diseases… The use of DDT in public health introduced ‘death control’ on a global scale, and, it is estimated that DDT has saved approximately 50 million lives and averted more than 1 billion human illnesses.”4 The enthusiasm that DDT generated in war time continued in peacetime. It was used everywhere, for control of every imaginable insect pest. It was considered harmless – children ran behind spray trucks to enjoy the cool spray - and remarkably few deaths can be attributed to it. In fact, there are no reports of accidental death due to DDT exposure: a remarkable record for any pesticide! Nonetheless, throughout the 1950s, evidence was mounting that DDT, as well as other new insecticides, had environmental consequences. The literature changes in tone from delighted awe at how long insecticidal activity persists to a simmering worry about finding residues in soil years after the last application of DDT or dieldrin. State environmental organizations – e.g., the Natural History Survey in Illinois, the Fish and Wildlife unit of California – documented disastrous effects on aquatic ecosystems after carefully controlled treatment of watersheds. Individual citizens – especially bird watchers – observed large bird kills after insecticide spraying, and, even more ominously, noticed that there were fewer and fewer birds of certain species. But most people in the US were unaware of the changing attitudes of the professionals or the concerns of the birders. When The New Yorker published Rachel Carson’s “Silent Spring” in 1962, the shock was enormous. Initially, opponents simply charged that Carson lied – that the evidence she presented was bogus. It is true that much of it was unpublished at the time; in fact, her book was a catalyst for many scientists to publish the data they had told her about, as well as for much additional research.5 4 RL Metcalf, :A century of DDT”, Journal of Agricultural and Food Chemistry 21:512, 1973. For at least a decade afterwards, people’s environmental attitudes could be identified by what they thought of this one book. Interestingly, pesticide defenders typically began with a half sentence praising the author of “Silent Spring”, following that with a “but” and vehement disagreement with her conclusions. 5 4 One of the earliest examples of ecosystem perturbation due to chlorinated hydrocarbon insecticides is described in the Bulletin of the World Health Organization 28:136-137, 19636. In Malaysia, spraying DDT against malaria mosquitos not only caused the roofs to collapse, but required an air-drop of cats because the village was threatened with plague. In this case, the DDT was being used in the World Health Organization's crusade against malaria, and effectively killed most of the mosquitos, achieving its goal of preventing the transmission of malaria. The spray incidentally also killed many other insects living in the village. Among these were the wasps that preyed on straw-eating moth larvae that inhabited the thatched roofs of the village. Without the wasps, the moths multiplied, and the roofs of the village collapsed. On the other hand, the roaches that inhabited the huts were relatively resistant to DDT, and accumulated high levels in their bodies without dying. Geckos - small tropical lizards that live in the thatch and eat assorted insects - are not resistant to DDT, and died from eating the DDT-laden roaches. Moreover, the dying geckos were easy prey for the village cats, which ingested toxic levels of DDT with the lizards. The cats, like the geckos, died. Rats began to multiply in the villages. Rats carry the fleas that transmit bubonic plague, so the World Health Organization had to fly in cats to prevent an epidemic of plague. Another early account of ecosystem perturbation came from Clear Lake, in northern California. Clear Lake, north of San Francisco, is a popular tourist area as well as a major breeding ground for the Western Grebe [Aechmophorus occidentalis], a particularly graceful, large water bird. It is also the home of the Clear Lake gnat (Chaoborus astictopus), an insect that is quite harmless, but so abundant that it is a major nuisance. Clear Lake was treated to control the gnats in 1949, 1954, and in 1957 at progressively higher levels of DDD, an insecticidal analog of DDT. It must be noted that the treatments were carried out according to the best pest management practices of the time, and with unusual care to avoid damage to the lake’s ecosystem: this was not a case of mismanagement. The results provide an excellent example of the interactions among species in an ecosystem7. After the 1949 spraying, water levels of DDD in the lake reached 14.2 ppb. No adverse effects were noted. DDD is a persistent insecticide, and gnat levels were suppressed for some years, but gradually increased again at Clear Lake. In 1954, DDD was again sprayed. Water levels of DDD reached 20 ppb, which, although higher than after the first spraying, are still trivial in terms of animal toxicity or even of insecticidal activity. There were grebe deaths noted after the 1954 spraying, but no illness was found to account for the deaths, and no connection to the DDD spraying was made. In 1957 gnat levels had again increased to the point that spraying was considered advisable (note the decreasing intervals between sprayings, which suggest that the gnat population was developing resistance to DDD). This third spraying led to massive grebe deaths. Before 1949, the Clear Lake grebe population had consisted of approximately 1,000 pairs of breeding birds; by 1954 it had decreased to 30 pairs, and even these produced no young. Again, no illnesses were found. When assays for DDD were carried out, water levels remained in the range of parts per billion. Levels in organisms were startlingly higher. DDD levels were 5 ppm in plankton, 40-300 ppm in plant-eating fish, and as high as 2500 ppm in the fat of My version of this story – like the Clear Lake story that follows – is taken from almost verbatim from my book, Toxic Substances in the Environment. However, the citation for Operation Cat Drop is wrong in the book – the correct citation is Bulletin of the World Health Organization 28:136-137, 1963. I took the Clear Lake story from Silent Spring, and Rachel Carson cites California Fish and Game 46(1):91-106, 1960 as her source. 6 7 See http://bioregion.ucdavis.edu/book/10_Clear_Lake/10_11_circ_cl_stpark.html for a synopsis of the Clear Lake story into the present. 5 one carnivorous fish. Grebes had up to 1600 ppm DDD in their fat. These data illustrate the concept of bioaccumulation: fat-soluble compounds (which are relatively water-insoluble compounds) that are not readily degraded will accumulate in fat and are found at increasing levels in organisms as one progresses up a food chain. The saga of the Clear Lake grebes was repeated many times in the 1950s and early 1960s. The insecticide was not always DDD or even its close analog DDT. Several other organochlorine insecticides had been developed to compete with DDT, and each was lauded for its long persistence in the field, which meant that farmers had to treat less often, saving time and money. Aldrin, dieldrin, chlordane, and heptachlor were the most heavily used of these compounds; endrin and endosulfan had more restricted use; mirex and chlordecone were used for a very few specific purposes. In time, each was shown to bioaccumulate and, eventually, each was banned, as described in the case history on the cyclodiene insecticides. Unfortunately, the lesson that was learned from the experience with DDD was not that either undue persistence or bioaccumulation was hazardous, but only that bioaccumulation was. Not until several organochlorine insecticides had been banned were factors other than bioaccumulation recognized as undesirable. 6